Question: Q.2. Van der Waals equation has a form: RT a P= V-b 72 1 In = CHIC RT -PdV - In Z+(Z-1) +2-1 V -plar
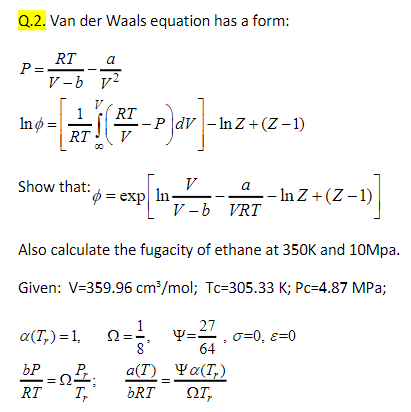
Q.2. Van der Waals equation has a form: RT a P= V-b 72 1 In = CHIC RT -PdV - In Z+(Z-1) +2-1 V -plar -- RT show that: p = exp In V a -- In Z+(Z-1) V-b VRT Also calculate the fugacity of ethane at 350K and 10Mpa. Given: V=359.96 cm/mol; Tc=305.33 K; Pc=4.87 MPa; g(,)=1, -16 bP 27 - VE O=0, E=0 64 a(1) Ya(T) DRT , : RT T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


