Question: Q3: A heater burns normal butane (nC4H10) using 40% excess air. Combustion is complete and the flue gas leaves the stack as - Shown in
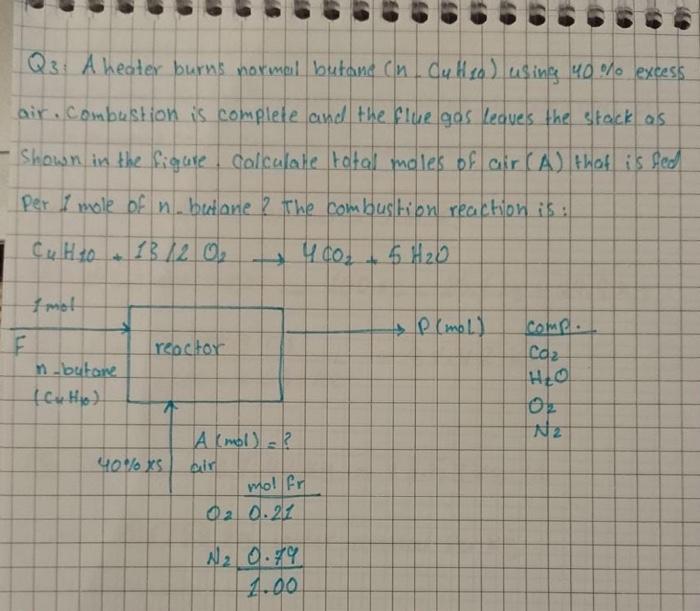
Q3: A heater burns normal butane (nC4H10) using 40% excess air. Combustion is complete and the flue gas leaves the stack as - Shown in the figure. calculate total moles of air (A) that is ged Per I mole of n-butane? The combustion reaction is: C4H10+13/2O24CO2+5H2O
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


