Question: Q3 A hydrogen atom is in its basic state. It is excited with a photon. a) Calculate the longest wavelength the photon can have if
Q3 A hydrogen atom is in its basic state. It is excited with a photon. a) Calculate the longest wavelength the photon can have if it is to be absorbed. b) Which orbital does the electron end up in absorption according to sub-task a)? Motivate! c) What is the ionization energy of the hydrogen atom in the ground state in kJ / mol?
Q4.
In this task, we start from the Hckel model. The secular determinant of ethylene -electrons have their roots 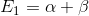 and
and 
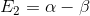
The roots have the corresponding secular determinant for benzene
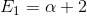 ,
, 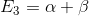 ,
, 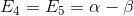 ,
, 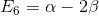 .
.
a).
Calculate the total the electron energy of ethylene in the ground state expressed in and
b)
Suppose you are now treating a benzene ion (a benzene molecule that has lost an electron). What will be the total the electron energy of the benzene ion in the ground state expressed in and ?
c)
Calculate the total the binding energy of the benzene ion in the ground state?
d)
What will be the delocalization energy for the benzene ion in the ground state?
e)
What will be the multiplicity of the benzene ion in the ground state? Motivate.
E = a +3 E2 = a - E1 = a + 2 E3 = a + 3 E4 E5 = a E6 = a - 28
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


