Question: Q4/ A liquid mixture is prepared by combining N different liquids with densities p1; 22; ...; Pn. The volume of component i added to the
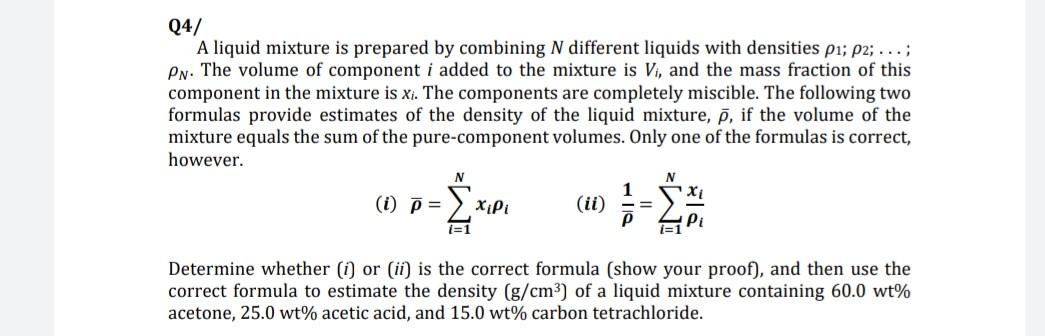
Q4/ A liquid mixture is prepared by combining N different liquids with densities p1; 22; ...; Pn. The volume of component i added to the mixture is Vi, and the mass fraction of this component in the mixture is xi. The components are completely miscible. The following two formulas provide estimates of the density of the liquid mixture, 7, if the volume of the mixture equals the sum of the pure-component volumes. Only one of the formulas is correct, however. N (i) 7 = (ii) 1=1 Determine whether (1) or (ii) is the correct formula (show your proof), and then use the correct formula to estimate the density (g/cm3) of a liquid mixture containing 60.0 wt% acetone, 25.0 wt% acetic acid, and 15.0 wt% carbon tetrachloride
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


