Question: Q.4 Formaldehyde is produced in a continuous reactor by oxidizing methane with pure oxygen. A side reaction is the combustion of methane to form carbon
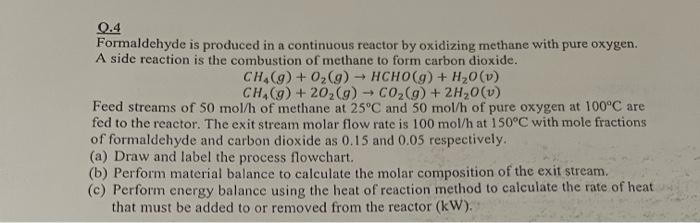
Q.4 Formaldehyde is produced in a continuous reactor by oxidizing methane with pure oxygen. A side reaction is the combustion of methane to form carbon dioxide. CH4(g)+O2(g)HCHO(g)+H2O(v)CH4(g)+2O2(g)CO2(g)+2H2O(v) Feed streams of 50mol/h of methane at 25C and 50mol/h of pure oxygen at 100C are fed to the reactor. The exit stream molar flow rate is 100mol/h at 150C with mole fractions of formaldehyde and carbon dioxide as 0.15 and 0.05 respectively. (a) Draw and label the process flowchart. (b) Perform material balance to calculate the molar composition of the exit stream. (c) Perform energy balance using the heat of reaction method to calculate the rate of heat that must be added to or removed from the reactor (kW)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


