Question: Q4. From the data plot found in Pre Lab question 4 in your lab manual, calculate the activation energy, Ea, for the reaction. 18.0kJ/mol 91.5kJ/mol
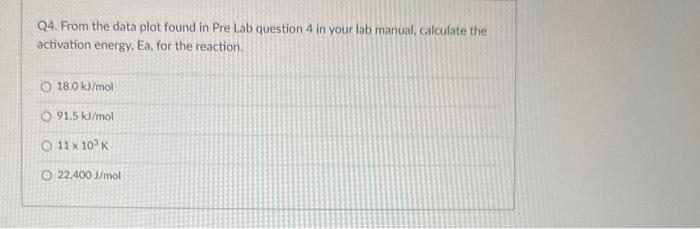
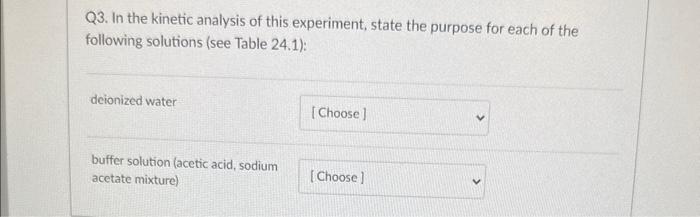
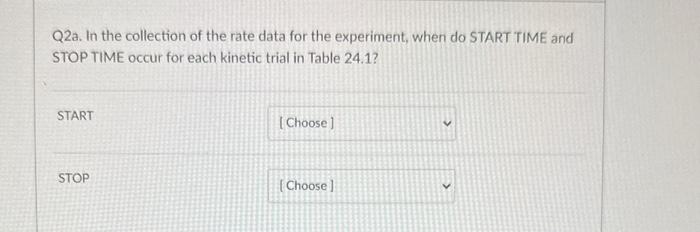
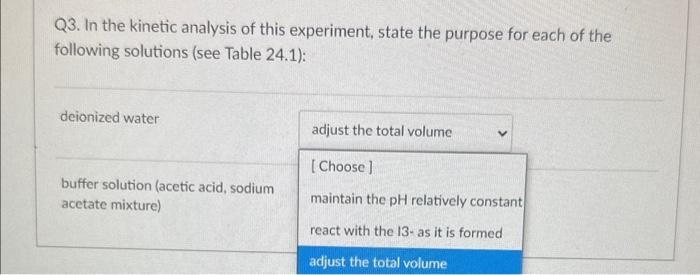
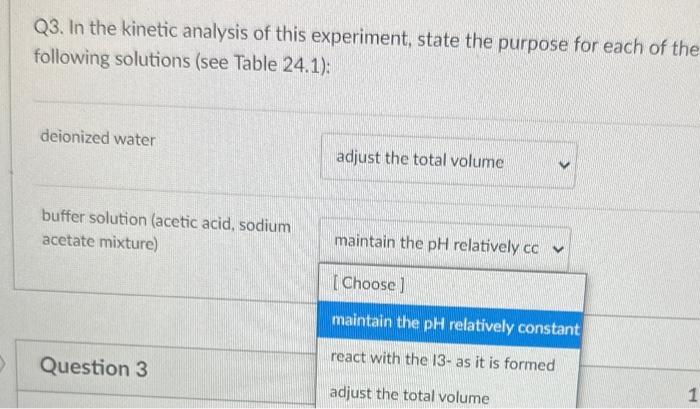
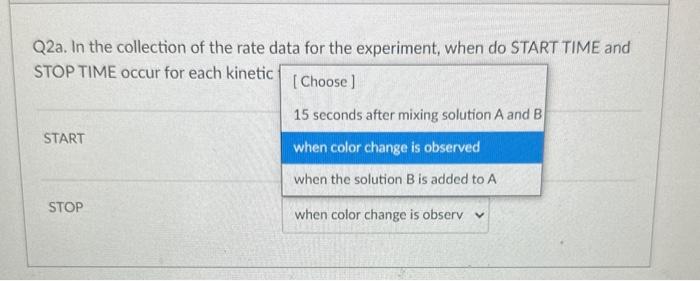
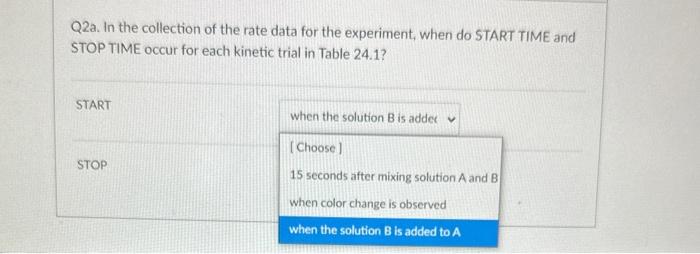
Q4. From the data plot found in Pre Lab question 4 in your lab manual, calculate the activation energy, Ea, for the reaction. 18.0kJ/mol 91.5kJ/mol 11103K 22,400J/mol Q3. In the kinetic analysis of this experiment, state the purpose for each of the following solutions (see Table 24.1): deionized water buffer solution (acetic acid, sodium acetate mixture) Q2a. In the collection of the rate data for the experiment, when do START TIME and STOP TIME occur for each kinetic trial in Table 24.1? START STOP Q3. In the kinetic analysis of this experiment, state the purpose for each of the following solutions (see Table 24.1): deionized water buffer solution (acetic acid, sodium acetate mixture) Q3. In the kinetic analysis of this experiment, state the purpose for each of the following solutions (see Table 24.1): deionized water buffer solution (acetic acid, sodium acetate mixture) Question 3 react with the 13-as it is formed adjust the total volume Q2a. In the collection of the rate data for the experiment, when do START TIME and STOP TIME occur for each kinetic START STOP Q2a. In the collection of the rate data for the experiment, when do START TIME and STOP TIME occur for each kinetic trial in Table 24.1? START when the solution B is addec v STOP Q4. From the data plot found in Pre Lab question 4 in your lab manual, calculate the activation energy, Ea, for the reaction. 18.0kJ/mol 91.5kJ/mol 11103K 22,400J/mol Q3. In the kinetic analysis of this experiment, state the purpose for each of the following solutions (see Table 24.1): deionized water buffer solution (acetic acid, sodium acetate mixture) Q2a. In the collection of the rate data for the experiment, when do START TIME and STOP TIME occur for each kinetic trial in Table 24.1? START STOP Q3. In the kinetic analysis of this experiment, state the purpose for each of the following solutions (see Table 24.1): deionized water buffer solution (acetic acid, sodium acetate mixture) Q3. In the kinetic analysis of this experiment, state the purpose for each of the following solutions (see Table 24.1): deionized water buffer solution (acetic acid, sodium acetate mixture) Question 3 react with the 13-as it is formed adjust the total volume Q2a. In the collection of the rate data for the experiment, when do START TIME and STOP TIME occur for each kinetic START STOP Q2a. In the collection of the rate data for the experiment, when do START TIME and STOP TIME occur for each kinetic trial in Table 24.1? START when the solution B is addec v STOP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


