Question: Q4: If few patients need 25% solution X, how many mL do you need of the 50% solution X to make 1000 mL of 25%
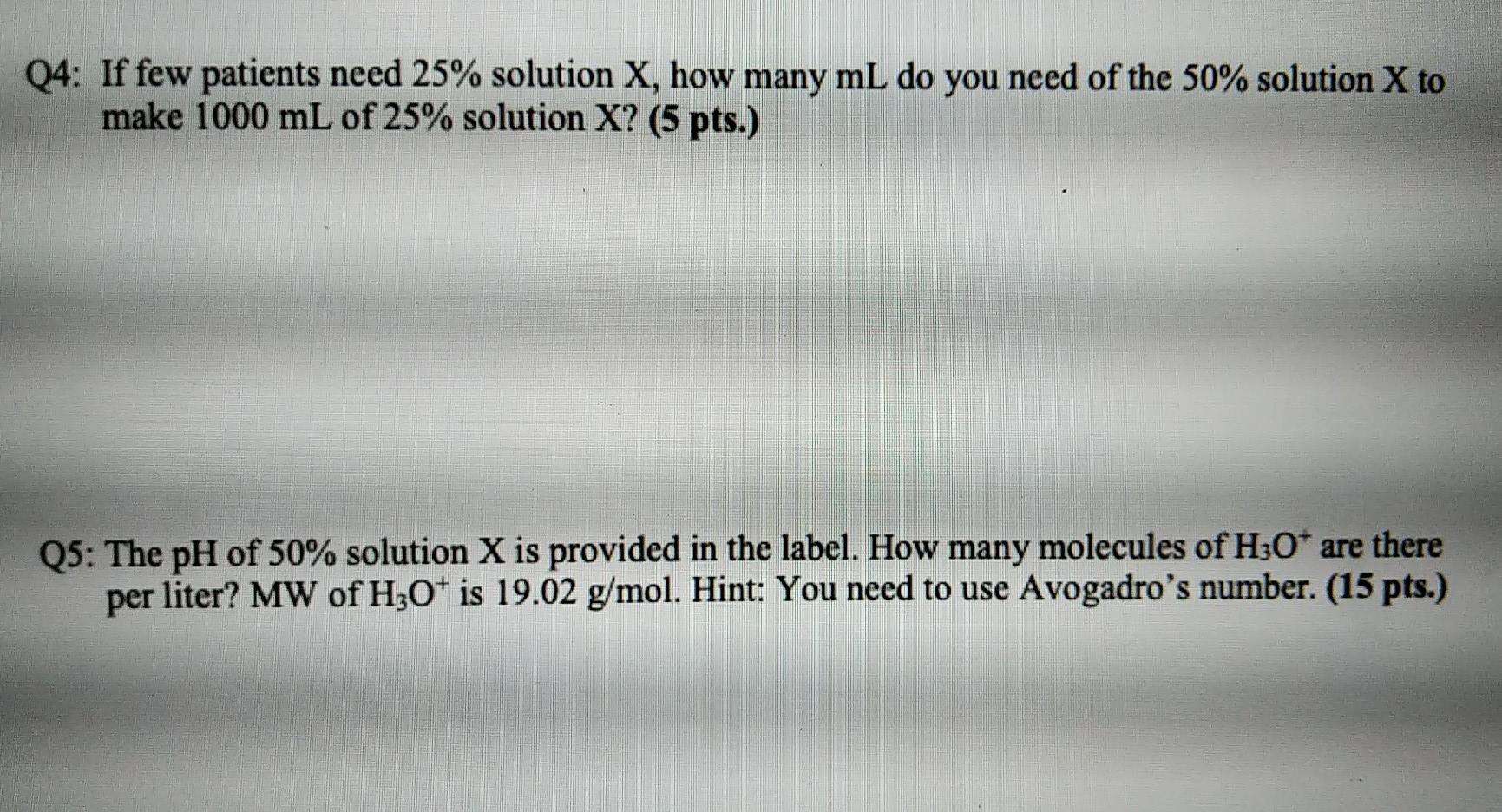
Q4: If few patients need 25% solution X, how many mL do you need of the 50% solution X to make 1000 mL of 25% solution X? (5 pts.) Q5: The pH of 50% solution X is provided in the label. How many molecules of H3O+ are there per liter? MW of H30+ is 19.02 g/mol. Hint: You need to use Avogadro's number. (15 pts.)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


