Question: Q6. (8 marks) Consider the graph below for liquid-vapor equilibrium for a binary system A and B. Temperature XA I. Label the regions of the
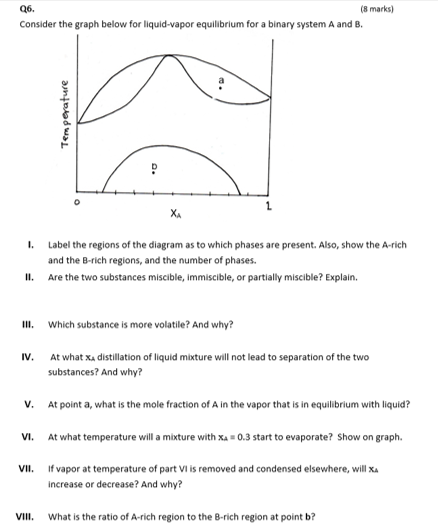
Q6. (8 marks) Consider the graph below for liquid-vapor equilibrium for a binary system A and B. Temperature XA I. Label the regions of the diagram as to which phases are present. Also, show the A-rich and the B-rich regions, and the number of phases. Are the two substances miscible, immiscible, or partially miscible? Explain. II. III. Which substance is more volatile? And why? IV. At what X, distillation of liquid mixture will not lead to separation of the two substances? And why? v. At point a, what is the mole fraction of A in the vapor that is in equilibrium with liquid? VI. At what temperature will a mixture with xa = 0.3 start to evaporate? Show on graph. VII. if vapor at temperature of part Vi is removed and condensed elsewhere, will XA increase or decrease? And why? VIII. What is the ratio of A-rich region to the B-rich region at point b? Q6. (8 marks) Consider the graph below for liquid-vapor equilibrium for a binary system A and B. Temperature XA I. Label the regions of the diagram as to which phases are present. Also, show the A-rich and the B-rich regions, and the number of phases. Are the two substances miscible, immiscible, or partially miscible? Explain. II. III. Which substance is more volatile? And why? IV. At what X, distillation of liquid mixture will not lead to separation of the two substances? And why? v. At point a, what is the mole fraction of A in the vapor that is in equilibrium with liquid? VI. At what temperature will a mixture with xa = 0.3 start to evaporate? Show on graph. VII. if vapor at temperature of part Vi is removed and condensed elsewhere, will XA increase or decrease? And why? VIII. What is the ratio of A-rich region to the B-rich region at point b
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


