Question: Q6. Answer this question using pressure-composition diagram for methanol(1) + ethanol(2) at 20 C: 90 80 70 p/ Torr 60 50 40 0.0 0.1 0.2
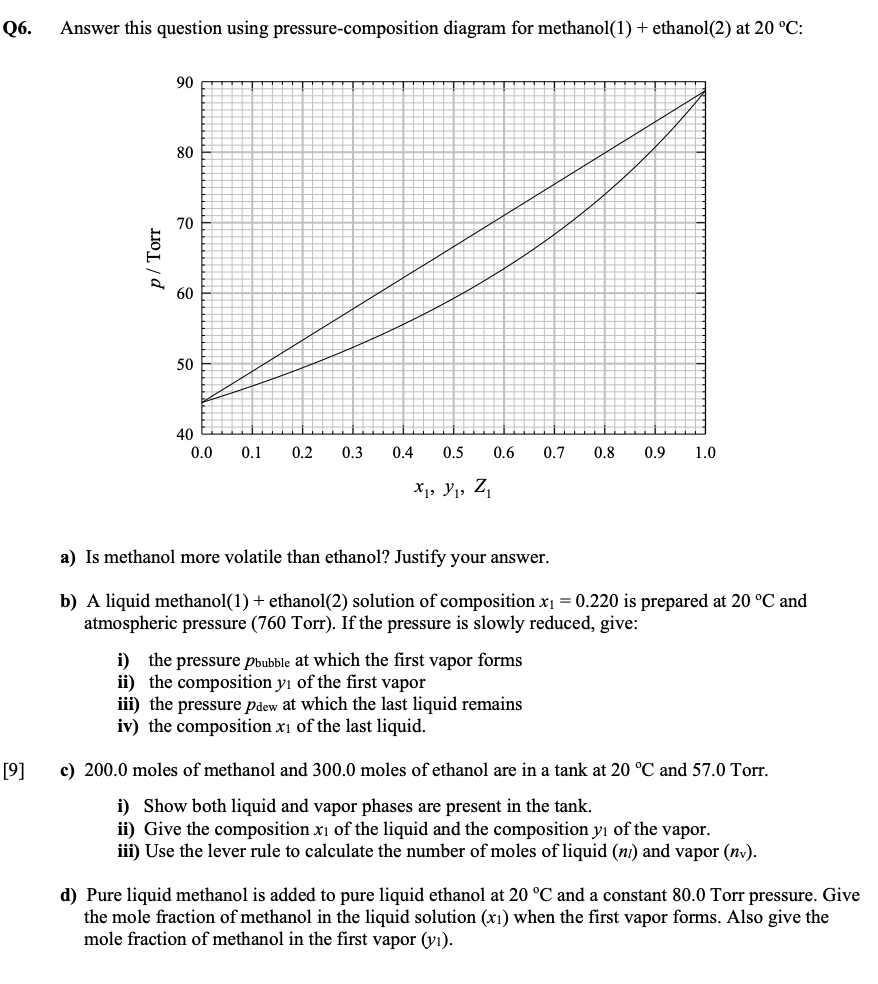
Q6. Answer this question using pressure-composition diagram for methanol(1) + ethanol(2) at 20 C: 90 80 70 p/ Torr 60 50 40 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 X, Y, Z a) Is methanol more volatile than ethanol? Justify your answer. b) A liquid methanol(1) + ethanol(2) solution of composition x1 = 0.220 is prepared at 20 C and atmospheric pressure (760 Torr). If the pressure is slowly reduced, give: i) the pressure Pbubble at which the first vapor forms ii) the composition y of the first vapor iii) the pressure pdew at which the last liquid remains iv) the composition xi of the last liquid. [9] c) 200.0 moles of methanol and 300.0 moles of ethanol are in a tank at 20 C and 57.0 Torr. i) Show both liquid and vapor phases are present in the tank. ii) Give the composition xi of the liquid and the composition y of the vapor. iii) Use the lever rule to calculate the number of moles of liquid (ni) and vapor (nv). d) Pure liquid methanol is added to pure liquid ethanol at 20 C and a constant 80.0 Torr pressure. Give the mole fraction of methanol in the liquid solution (xi) when the first vapor forms. Also give the mole fraction of methanol in the first vapor (yi)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


