Question: Q7. When measured with a 1.00-cm cell, an 8.50 x 10-5 M solution of species A exhibited absorbances of 0.129 and 0.764 at 475 and
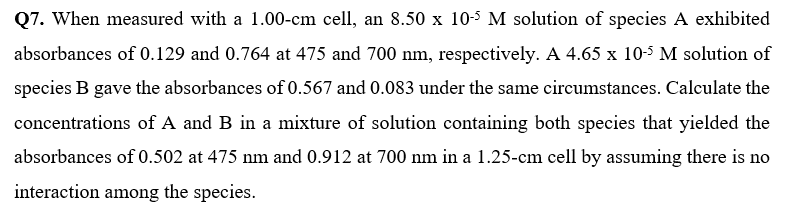
Q7. When measured with a 1.00-cm cell, an 8.50 x 10-5 M solution of species A exhibited absorbances of 0.129 and 0.764 at 475 and 700 nm, respectively. A 4.65 x 10-5 M solution of species B gave the absorbances of 0.567 and 0.083 under the same circumstances. Calculate the concentrations of A and B in a mixture of solution containing both species that yielded the absorbances of 0.502 at 475 nm and 0.912 at 700 nm in a 1.25-cm cell by assuming there is no interaction among the species
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


