Question: Q9,Q8 Write a net ionic equation for the reaction that occurs when excess hydroiodic acid and potassium carbonate(aq) are combined. (Be sure to specify states

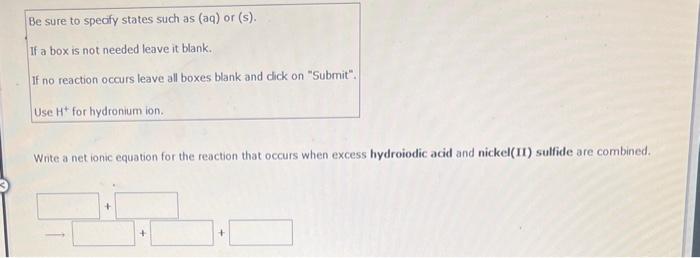
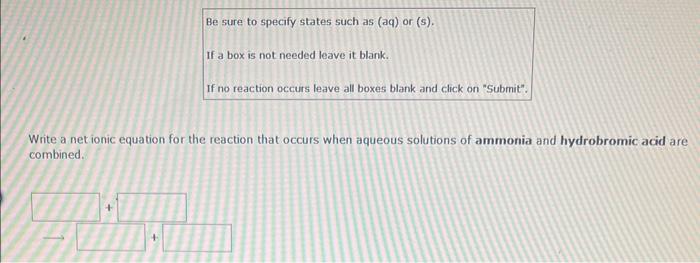
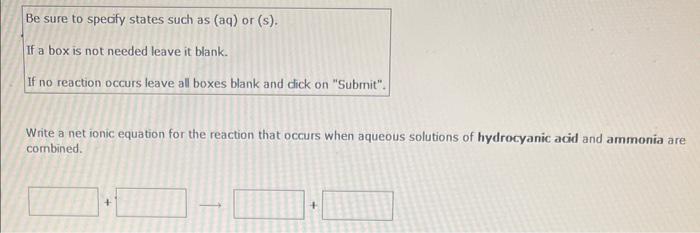
Write a net ionic equation for the reaction that occurs when excess hydroiodic acid and potassium carbonate(aq) are combined. (Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "submit". Use H+for hydronium ion.) Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and dick on "Submit". Use H+for hydronium ion. Write a net ionic equation for the reaction that occurs when excess hydroiodic acid and nickel(II) sulfide are combined. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "Submit". Write a net ionic equation for the reaction that occurs when aqueous solutions of ammonia and hydrobromic acid are combined. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and dick on "Submit". Write a net ionic equation for the reaction that occurs when aqueous solutions of hydrocyanic acid and ammonia are combined
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


