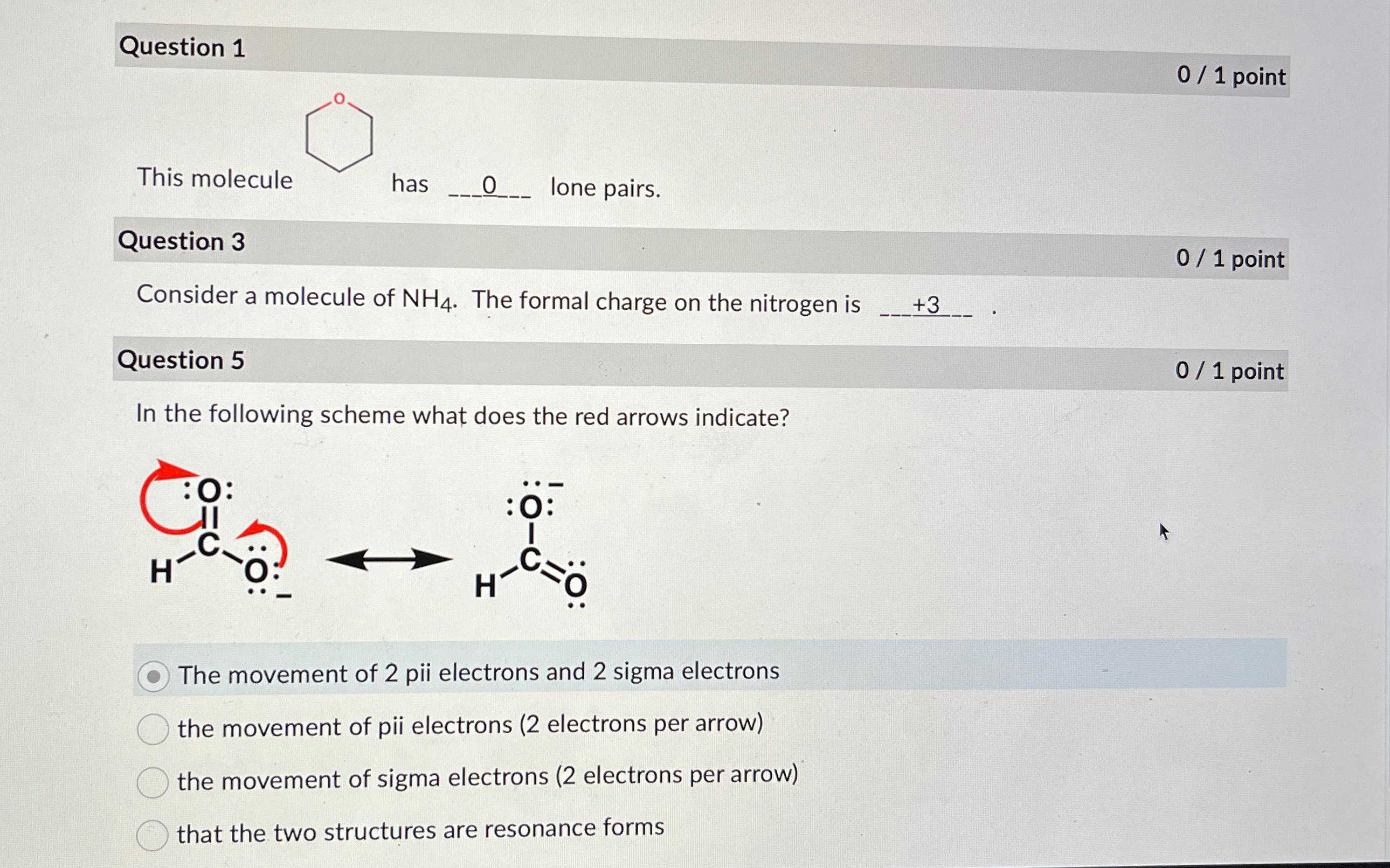
Question: Question 1 0 1 point This molecule has 0 ? lone pairs. Question 3 0 1 point Consider a molecule of N H 4 .
Question
point
This molecule has lone pairs.
Question
point
Consider a molecule of The formal charge on the nitrogen is
Question
point
In the following scheme what does the red arrows indicate?
The movement of pii electrons and sigma electrons
the movement of pii electrons electrons per arrow
the movement of sigma electrons electrons per arrow
that the two structures are resonance forms
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


