Question: Question 1 (1 point) Select the best statement in order to complete the model. Hydrogen Atom Oxygen Atom Carbon Atom Methane Oxygen Molecule Carbon Dioxide
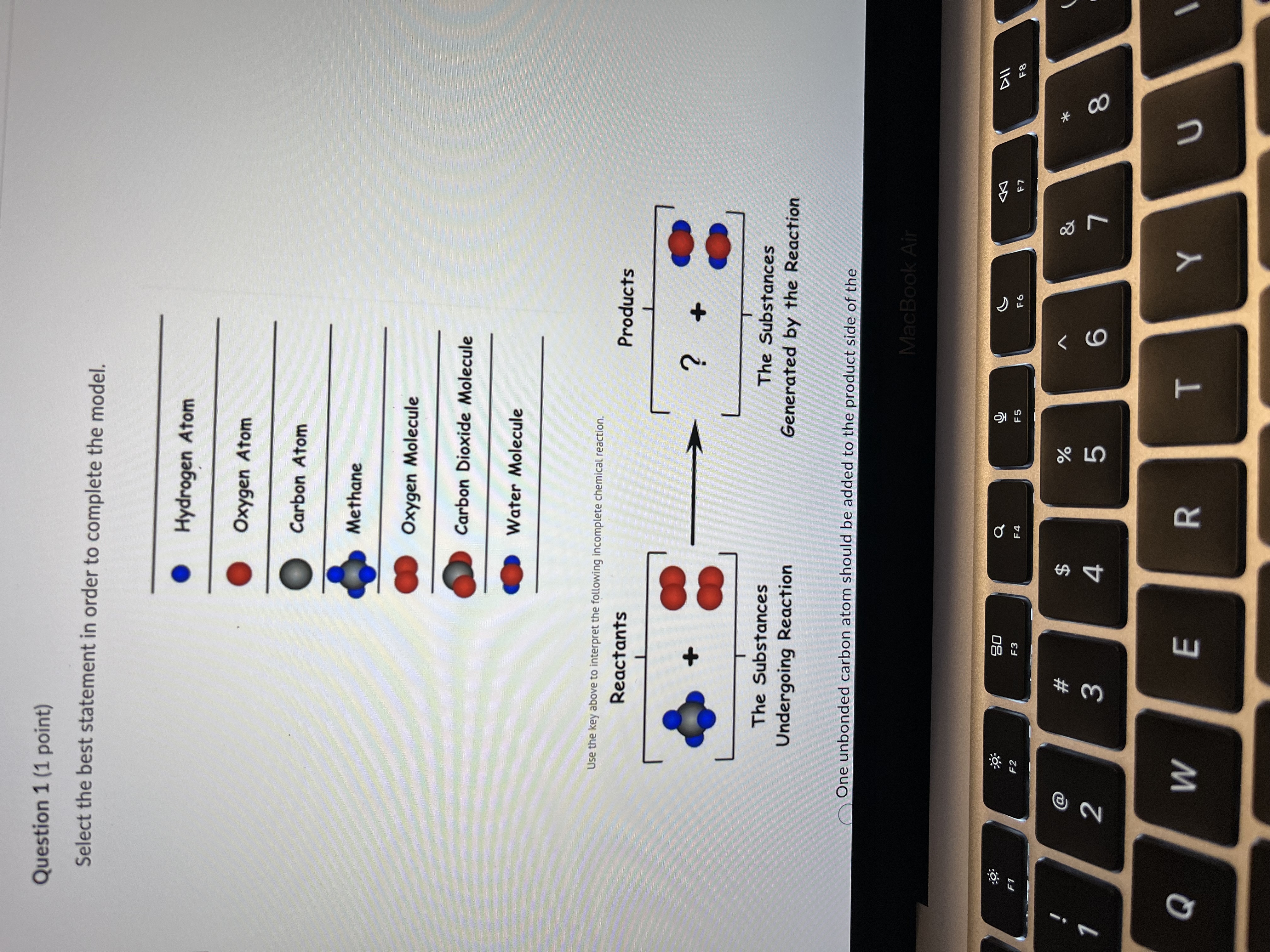
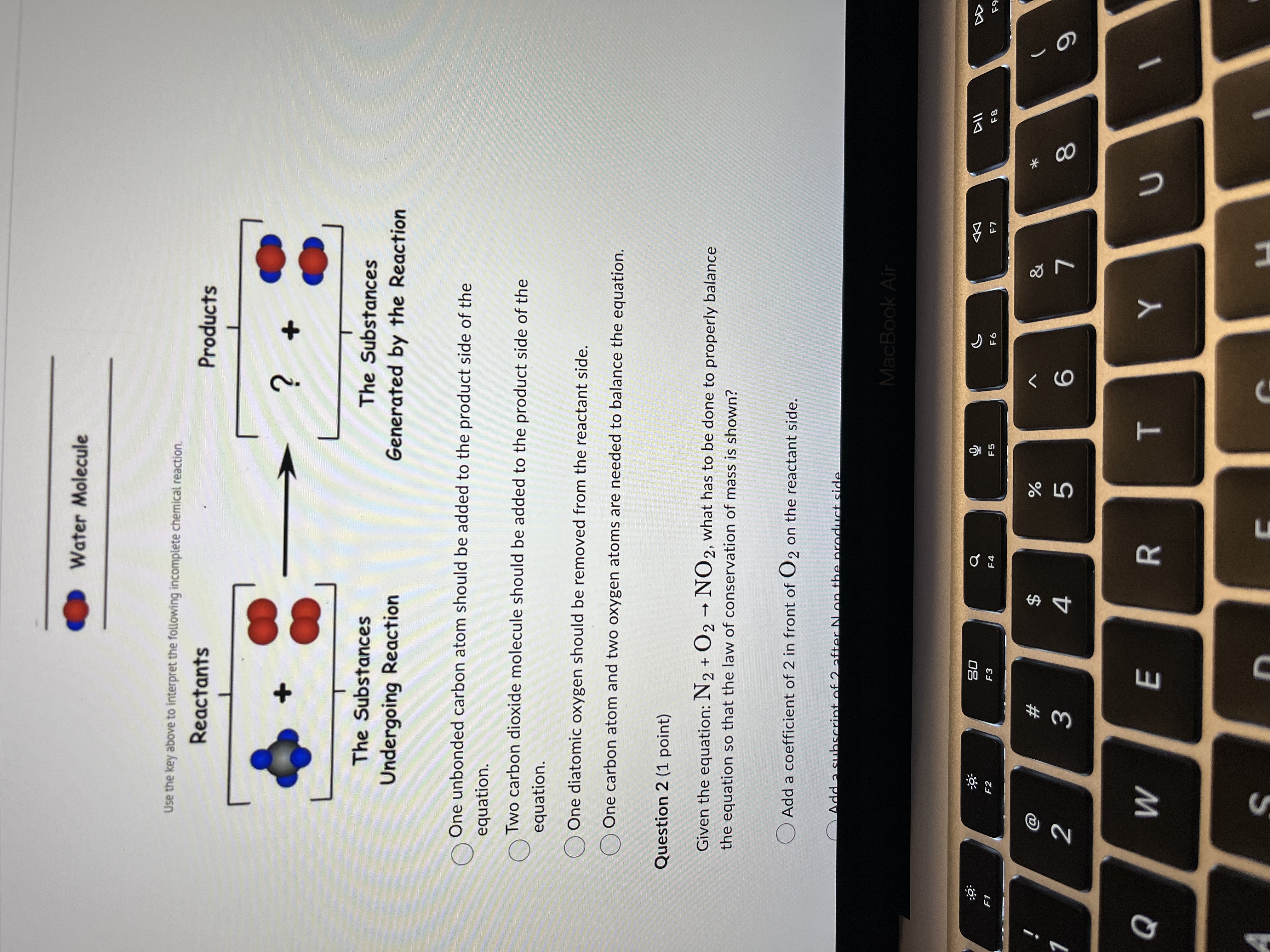
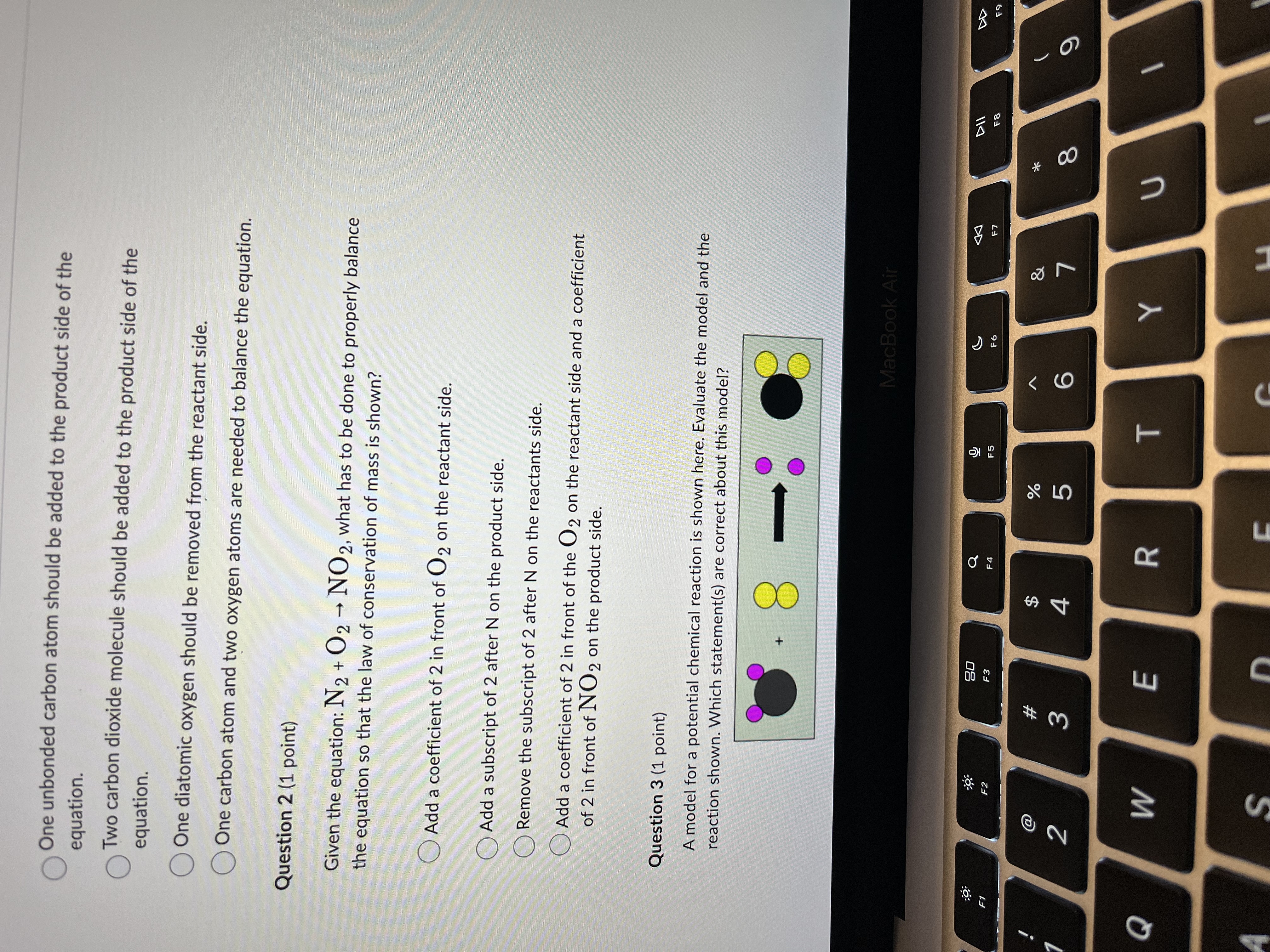


Question 1 (1 point) Select the best statement in order to complete the model. Hydrogen Atom Oxygen Atom Carbon Atom Methane Oxygen Molecule Carbon Dioxide Molecule Water Molecule Use the key above to interpret the following incomplete chemical reaction. Reactants Products ? The Substances The Substances Undergoing Reaction Generated by the Reaction One unbonded carbon atom should be added to the product side of the MacBook Air DII :9 20 G F5 F6 F8 F1 F2 F3 F4 @ $ % & * # 2 3 4 5 6 8 Q W E R T YWater Molecule Use the key above to interpret the following incomplete chemical reaction. Reactants Products 2 The Substances The Substances Undergoing Reaction Generated by the Reaction One unbonded carbon atom should be added to the product side of the equation. O Two carbon dioxide molecule should be added to the product side of the equation. One diatomic oxygen should be removed from the reactant side. One carbon atom and two oxygen atoms are needed to balance the equation. Question 2 (1 point) Given the equation: N2 + 02 - NO 2, what has to be done to properly balance the equation so that the law of conservation of mass is shown? Add a coefficient of 2 in front of O2 on the reactant side. Add 2 subscript MacBook Air 20 G 44 F1 F2 F3 F6 F7 $ & # 3 4 5 6 8 9 Q W E R T Y UOne unbonded carbon atom should be added to the product side of the equation. Two carbon dioxide molecule should be added to the product side of the equation. One diatomic oxygen should be removed from the reactant side. One carbon atom and two oxygen atoms are needed to balance the equation. Question 2 (1 point) Given the equation: N2 + 02 - NO 2, what has to be done to properly balance the equation so that the law of conservation of mass is shown? Add a coefficient of 2 in front of O2 on the reactant side. Add a subscript of 2 after N on the product side. Remove the subscript of 2 after N on the reactants side. Add a coefficient of 2 in front of the O2 on the reactant side and a coefficient of 2 in front of NO 2 on the product side. Question 3 (1 point) A model for a potential chemical reaction is shown here. Evaluate the model and the reaction shown. Which statement(s) are correct about this model? + 8 MacBook Air DII DD 20 FO F1 F2 F3 F6 F7 F8 $ & # 2 3 4 5 6 8 9 Q W E R T UAdd a subscript of 2 after N on the product side. Remove the subscript of 2 after N on the reactants side. Add a coefficient of 2 in front of the O2 on the reactant side and a coefficient of 2 in front of NO 2 on the product side. Question 3 (1 point) A model for a potential chemical reaction is shown here. Evaluate the model and the reaction shown. Which statement(s) are correct about this model? Select 1 correct answer(s) Conservation of matter is not observed because there are different numbers of molecules in the reactants and the products. Conservation of matter is not observed because the atoms are rearranged from one side of the reaction to the other. O Conservation of matter is not observed because some of the atoms are converted into energy in the reaction. Conservation of matter is observed because there are the same number of each atom on both sides of the reaction. Question 4 (1 point) 3 HCIO --> 2 HCIO2 + HCI Which of the following statements best describes the above chemical equation? MacBook Air DII DO 20 F7 F8 F9 F1 F2 F3 EA F6 a $ % # 5 8 9 2 3 4 6 Q W E R TSelect 1 correct answer(s) Conservation of matter is not observed because there are different numbers of molecules in the reactants and the products. O Conservation of matter is not observed because the atoms are rearranged from one side of the reaction to the other. O Conservation of matter is not observed because some of the atoms are converted into energy in the reaction. Conservation of matter is observed because there are the same number of each atom on both sides of the reaction. Question 4 (1 point) 3 HCIO --> 2 HCIO2 + HCI Which of the following statements best describes the above chemical equation? No. The number of O atoms is not balanced. Yes, there are the same number of atoms of each element on both the reactant and product sides of the equation. No. The total number of H atoms is not balanced. No. The total number of CI atoms is not balanced. Question 5 (1 point) Which of the following chemical equations is INCORRECTLY balanced? 04 Na + 02 - 2 Na2 0 O2 Al + 2 H2 SO4 - Al2(SO4) 3 + 3 H2 MacBook Air DD 30 EA F5 F6 F7 F8 F9 F1 F2 F3 @ # $ % & 4 5 6 8 9 N 3 Q W E R T Y UAttempt 1 Question 5 (1 point) Which of the following chemical equations is INCORRECTLY balanced? 04 Na + 02 - 2 Na2 0 2 Al + 2 H2 SO4 - Al2(SO4)3 + 3 H2 OCu + 2 AgNO3 - Cu(NO3)2 + 2 Ag OZn + 2 HCI - ZnCl2 + H2 Question 6 (5 points) Match the terms with the expressions listed. Endothermic and exothermic 26 grams of NaCl created from 13 grams of Na and 13 grams of CI. 1. Conservation of Energy Mass of reactants = mass of 2. Conservation of Mass products 3. Both Can't be created or destroyed Energy of the reactions = Energy of products MacBook Air 30 G DII E6 F7 F8 F9 F1 F3 $ & @ # 9 2 3 4 5 6 Q W E R T Y S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


