Question: Question 1 = 12 If a flask is initially filled with PH, = P = 4.7 atm at 425 C, calculate the partial pressure of
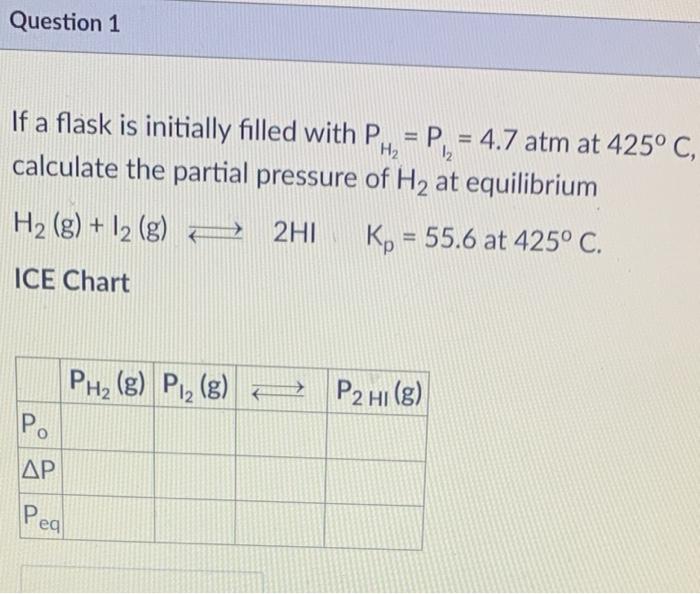
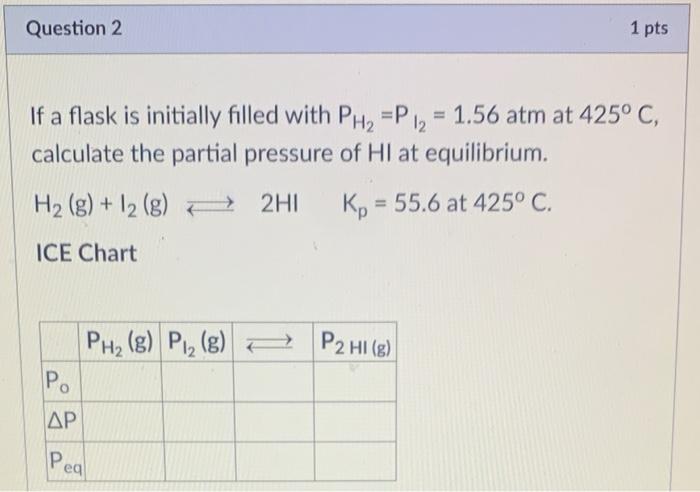
Question 1 = 12 If a flask is initially filled with PH, = P = 4.7 atm at 425 C, calculate the partial pressure of H2 at equilibrium H2(g) + 12 (g) 2HI Kp = 55.6 at 425 C. ICE Chart PHz (g) P2 (g) P2 HI(g) P. AP Peal Question 2 1 pts If a flask is initially filled with PH2 =P 12 = 1.56 atm at 425C, calculate the partial pressure of Hl at equilibrium. H2 (g) + 12 (g) + 2HI Kp = 55.6 at 425C. = ICE Chart PH2(g) Pl_ (g) = P2 HI(g) Po . Peal
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


