Question: Question 1 (2 points) Fill in the appropriate values for each blank as it refers to ATOM 1. The number of protons present in ATOM
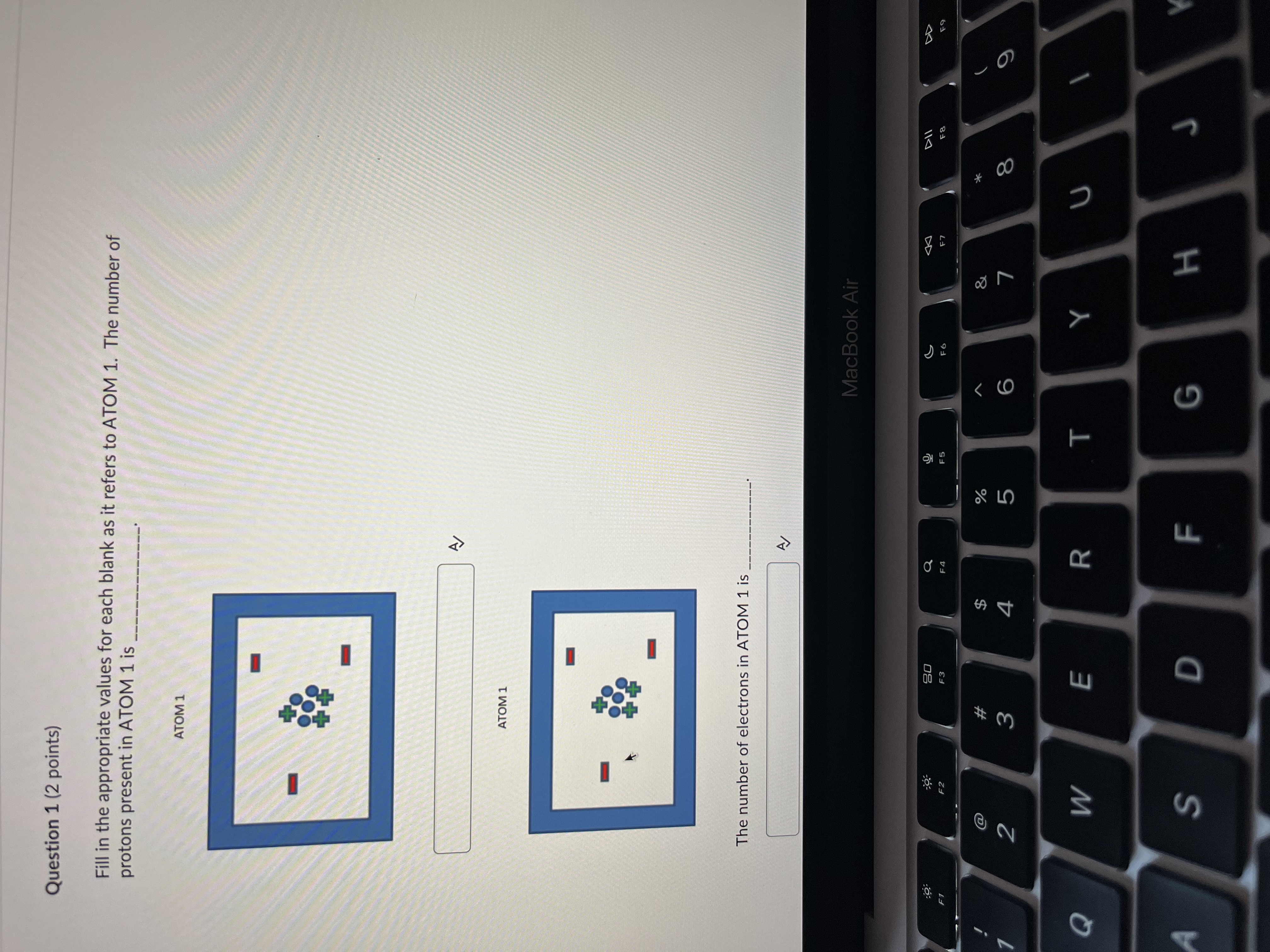
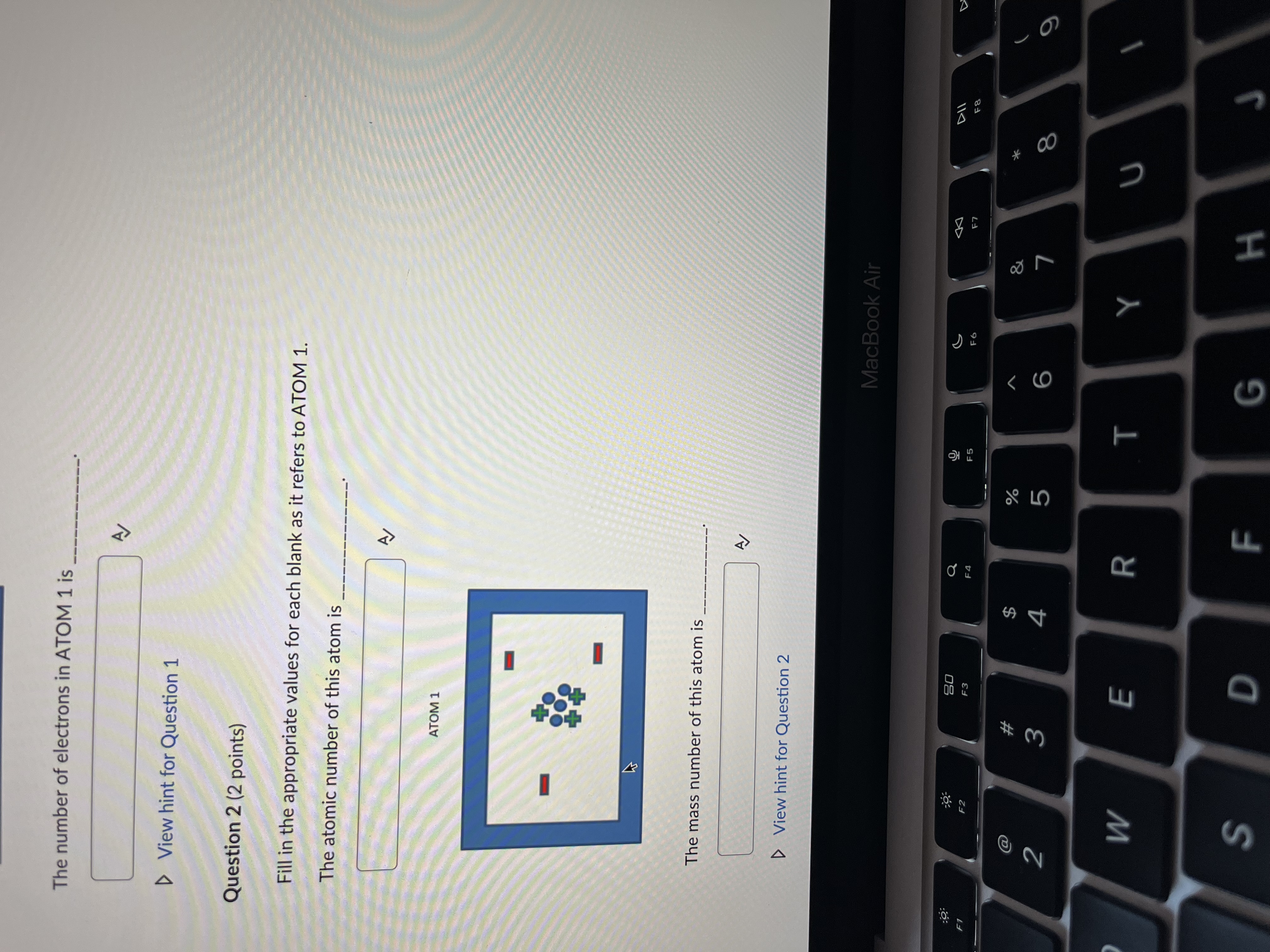
Question 1 (2 points) Fill in the appropriate values for each blank as it refers to ATOM 1. The number of protons present in ATOM 1 is ATOM 1 ATOM 1 The number of electrons in ATOM 1 is MacBook Air DD 20 S F8 FO 53 X @ # 8 2 3 4 5 9 Q W E R T Y U A S D F G HThe number of electrons in ATOM 1 is D View hint for Question 1 Question 2 (2 points) Fill in the appropriate values for each blank as it refers to ATOM 1. The atomic number of this atom is ATOM 1 The mass number of this atom is A D View hint for Question 2 MacBook Air 20 Q G DII F1 F2 F3 F5 F6 F7 F8 @ # & 2 3 4 5 6 W E R T Y U S D F G HThe mass number of this atom is A/ D View hint for Question 2 Question 3 (1 point) A neutral atom has 14 protons and 18 neutrons. Choose the correct nuclide symbol for this atom (1 point). a. 3218 Ar b. $214 Si C. 14 Si d. 1432 Ge A OB Oc OD Question 4 (1 point) Carbon-12 and Carbon-14 are considered of each other. (1 point) > View hint for Question 4 MacBook Air 20 G DII DD F2 F3 F6 F7 F8 F9 # % 3 4 5 6 9 W E R T Y U S D F G H KUC OD Question 4 (1 point) Carbon-12 and Carbon-14 are considered of each other. (1 point) D View hint for Question 4 Question 5 (4 points) There are three stable atoms of Argon (Atomic Number 18): Argon-36, Argon-38 and Argon-40. What would the atoms of these isotopes have in common? What would be different about their atoms? (4 points) A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


