Question: QUESTION 1 : ( 3 5 pts ) : ( a ) ( 6 pts ) Describe an experiment from which you can estimate the
QUESTION : pts:
a pts Describe an experiment from which you can estimate the Flory interaction parameter, for a given polymer and solvent?
b pts FloryHuggins theory considers only the attractive interactions between the molecules, therefore, the interaction parameter has only the enthalpy part. Due to volume change upon mixing, there is also entropy contribution to Experimentally, it was shown that where is the volume of the molecules What is the largest solubility parameter difference that allows polymer solutions with polymer's degree of polymerization to be miscible at room temperature? Take Boltzmann constant,
c pts Based on your finding, what solvents listed in Table attached can dissolve polystyrene with degree of polymerization
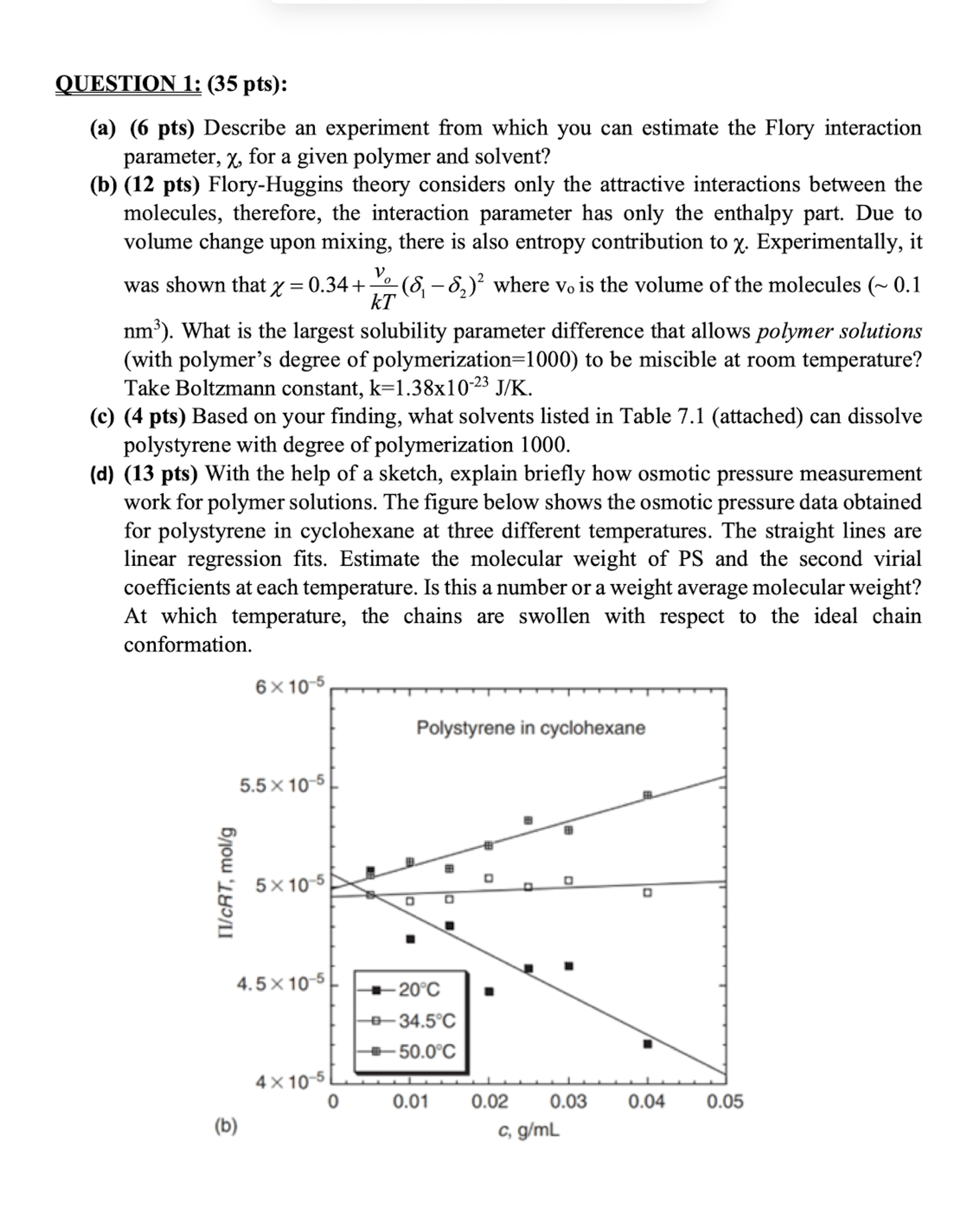
d pts With the help of a sketch, explain briefly how osmotic pressure measurement work for polymer solutions. The figure below shows the osmotic pressure data obtained for polystyrene in cyclohexane at three different temperatures. The straight lines are linear regression fits. Estimate the molecular weight of PS and the second virial coefficients at each temperature. Is this a number or a weight average molecular weight? At which temperature, the chains are swollen with respect to the ideal chain conformation.Describe an experiment from which you can estimate the Flory interaction
parameter, chi for a given polymer and solvent?
b pts FloryHuggins theory considers only the attractive interactions between the
molecules, therefore, the interaction parameter has only the enthalpy part. Due to
volume change upon mixing, there is also entropy contribution to chi Experimentally, it
was shown that
v where vo is the volume of the molecules ~
o
kT
nm What is the largest solubility parameter difference that allows polymer solutions
with polymers degree of polymerization to be miscible at room temperature?
Take Boltzmann constant, kx JK
c pts Based on your finding, what solvents listed in Table attached can dissolve
polystyrene with degree of polymerization
d pts With the help of a sketch, explain briefly how osmotic pressure measurement
work for polymer solutions. The figure below shows the osmotic pressure data obtained
for polystyrene in cyclohexane at three different temperatures. The straight lines are
linear regression fits. Estimate the molecular weight of PS and the second virial
coefficients at each temperature. Is this a number or a weight average molecular weight?
At which temperature, the chains are swollen with respect to the ideal chain
conformation.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


