Question: Question 1 (30 points) A 3D infinite quantum well is a very simple model for an atom. Suppose that two cubic 3D infinite quantum
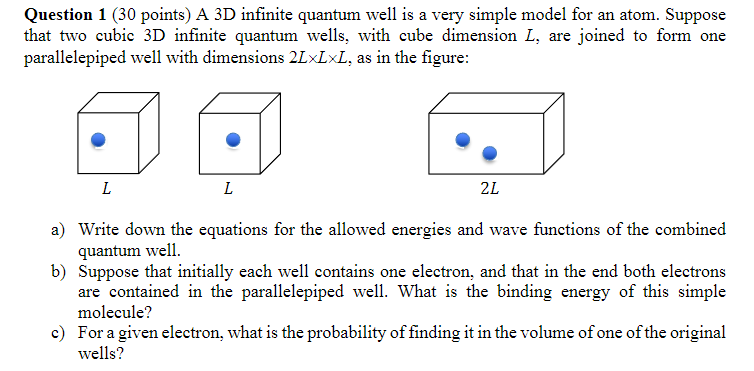
Question 1 (30 points) A 3D infinite quantum well is a very simple model for an atom. Suppose that two cubic 3D infinite quantum wells, with cube dimension L, are joined to form one parallelepiped well with dimensions 2LxLxL, as in the figure: 2L a) Write down the equations for the allowed energies and wave functions of the combined quantum well. b) Suppose that initially each well contains one electron, and that in the end both electrons are contained in the parallelepiped well. What is the binding energy of this simple molecule? c) For a given electron, what is the probability of finding it in the volume of one of the original wells?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


