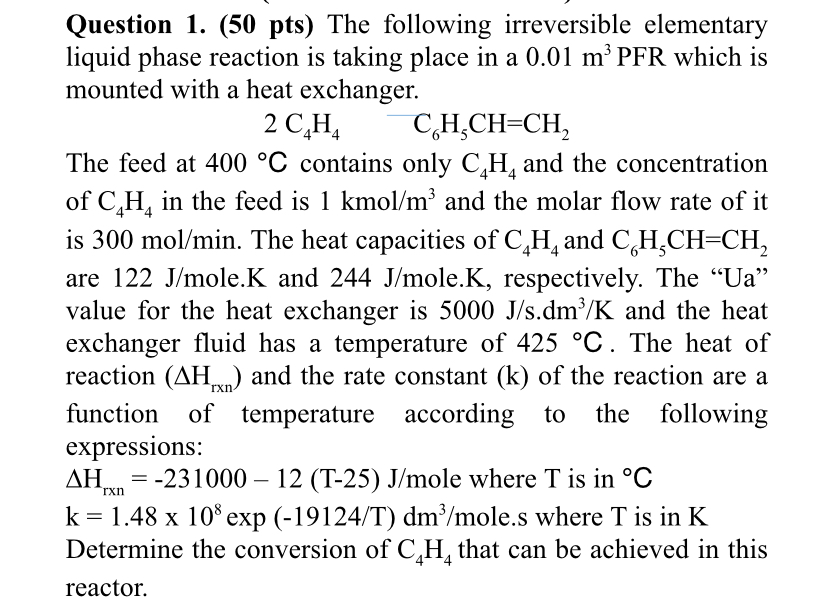
Question: Question 1 . ( 5 0 pts ) The following irreversible elementary liquid phase reaction is taking place in a 0 . 0 1 m
Question pts The following irreversible elementary
liquid phase reaction is taking place in a which is
mounted with a heat exchanger.
The feed at contains only and the concentration
of in the feed is kmo and the molar flow rate of it
is The heat capacities of and
are ole. and ole. respectively. The Ua
value for the heat exchanger is and the heat
exchanger fluid has a temperature of The heat of
reaction and the rate constant of the reaction are a
function of temperature according to the following
expressions:
mole where is in
expole. where is in
Determine the conversion of that can be achieved in this
reactor.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


