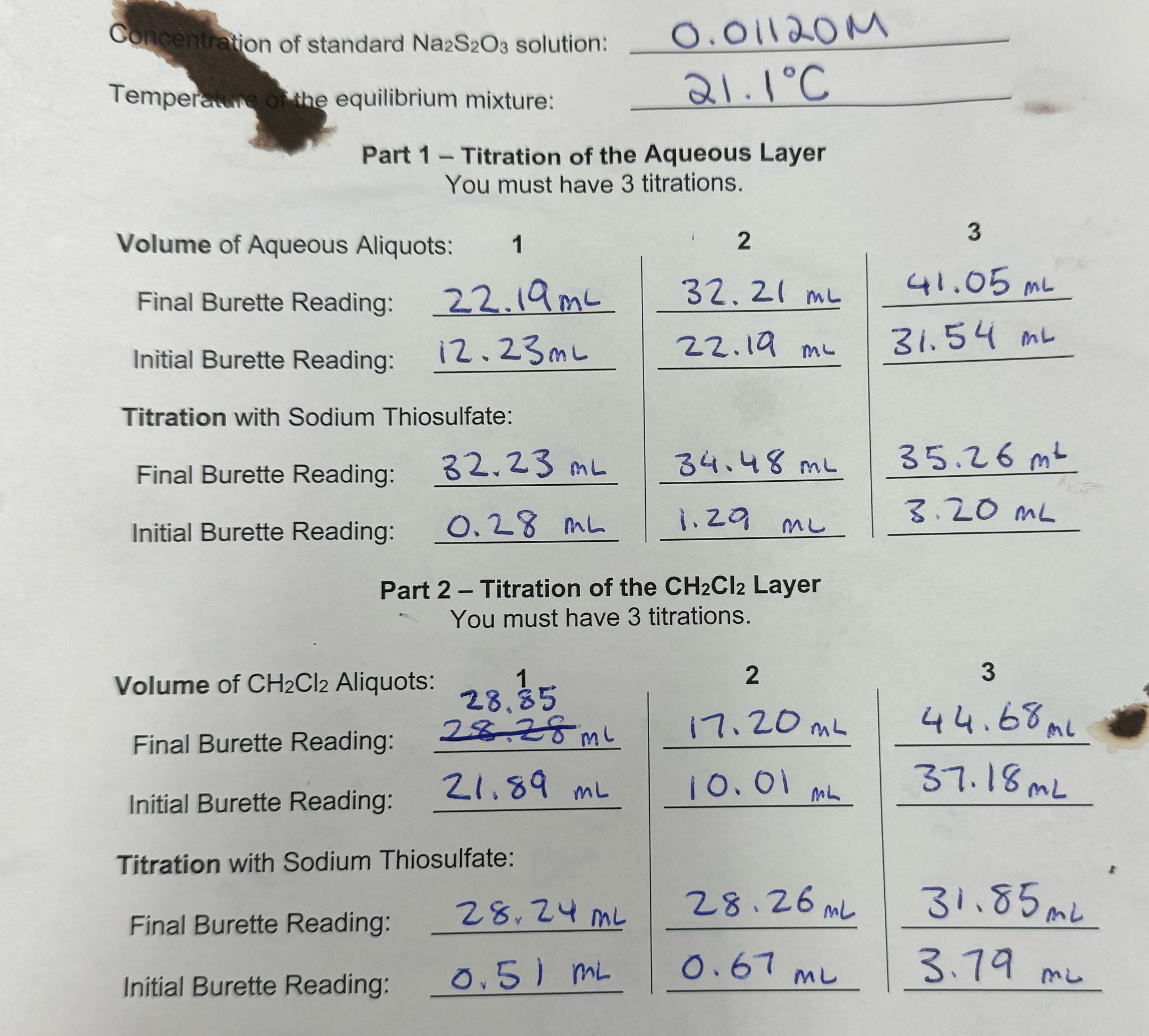
Question: Question 1 7 ( 1 point ) Saved Using your own lab - obtained data, what was your calculated K C in water? Report your
Question point
Saved
Using your own labobtained data, what was your calculated in water? Report your answer to the nearest whole number.
Concentration of standard solution:
Tempera the equilibrium mixture:
Part Titration of the Aqueous Layer
You must have titrations.
Volume of Aqueous Aliquots:
Final Burette Reading:
Initial Burette Reading:
Titration with Sodium Thiosulfate:
tableFinal Burette Reading:,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


