Question: Question 1: A) Given the reaction below, please write the equilibrium constant equations for the forward AND reverse reactions. A(g)+B(g)2C(g) FORWARD: REVERSE: B) A scientist
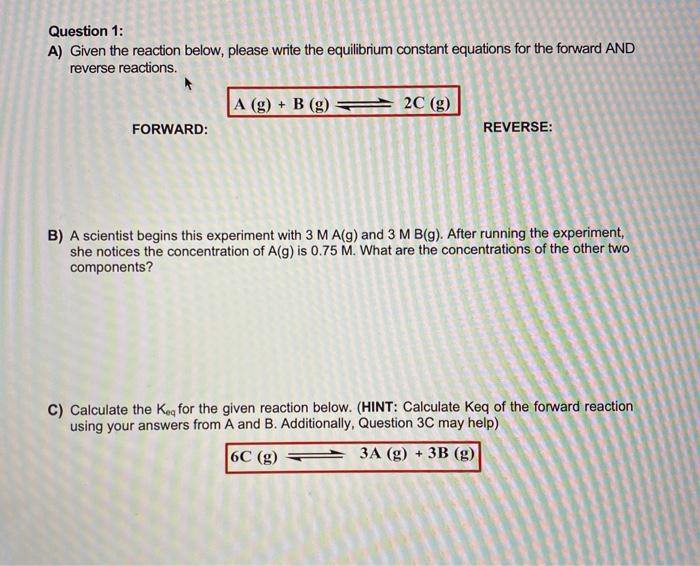
Question 1: A) Given the reaction below, please write the equilibrium constant equations for the forward AND reverse reactions. A(g)+B(g)2C(g) FORWARD: REVERSE: B) A scientist begins this experiment with 3MA(g) and 3MB(g). After running the experiment, she notices the concentration of A(g) is 0.75M. What are the concentrations of the other two components? C) Calculate the Keq for the given reaction below. (HINT: Calculate Keq of the forward reaction using your answers from A and B. Additionally, Question 3C may help) 6C(g)3A(g)+3B(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


