Question: Question 1 Calculate the split fraction, split ratio, separation power and purity of each column. Feed G'rich ingrich Table 1.5 Material Balance for Hydrocarbon Recovery
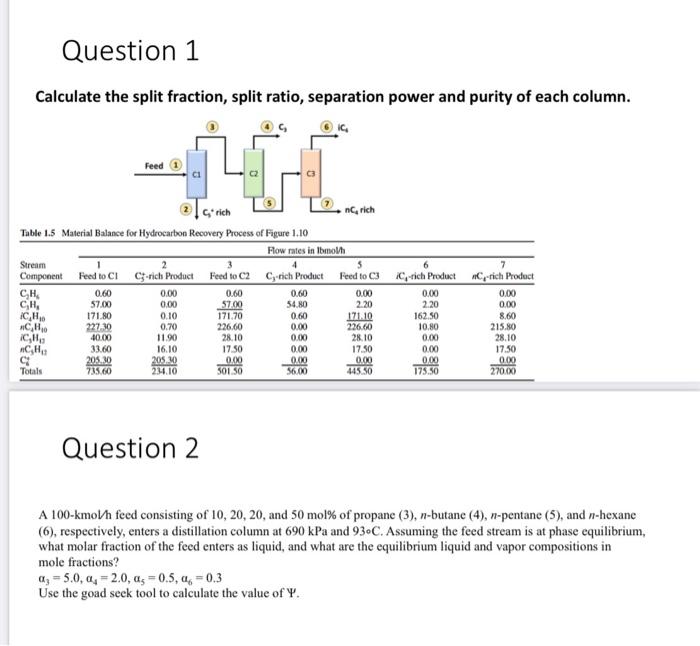
Question 1 Calculate the split fraction, split ratio, separation power and purity of each column. Feed G'rich ingrich Table 1.5 Material Balance for Hydrocarbon Recovery Process of Figure 1.10 Flow rates in Ibmoth Stream 2 3 4 $ Component Feed to CI C-rich Product Feed to C2 C, rich Product Feed to C3 0.60 0.00 0.60 0.60 0.00 CH, 57.00 0.00 57.00 54.80 2.20 ICH 171.80 0.10 171.70 0.60 171.10 227.30 0.70 226,60 0.00 226.60 40.00 11.90 28.10 0.00 28.10 33.60 16.10 1750 0.00 17.50 C 205.10 205.00 0.00 0.000 0.00 Totals 735.60 234.10 56.00 445.50 CH 6 IC-rich Product 0.00 2.20 162.50 10.80 0.00 0.00 0.00 175.50 7 C-rich Product 0.00 0.00 8.60 215.80 28.10 1750 0.00 270.00 CH ICH CHA 501 50 Question 2 A 100-kmoVh feed consisting of 10, 20, 20, and 50 mol% of propane (3), n-butane (4), n-pentanc (5), and n-hexane (6), respectively, enters a distillation column at 690 kPa and 93.C. Assuming the feed stream is at phase equilibrium, what molar fraction of the feed enters as liquid, and what are the equilibrium liquid and vapor compositions in mole fractions? a: = 5.0,q, = 2.0, as=0.5,44 -0,3 Use the goad seek tool to calculate the value of Y
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


