Question: Question 1 CHA+20, +CO +2H30 Identify the limiting reagent for the reaction of 64.00 g of methane pas roncting with the reaction of 61.0 pm
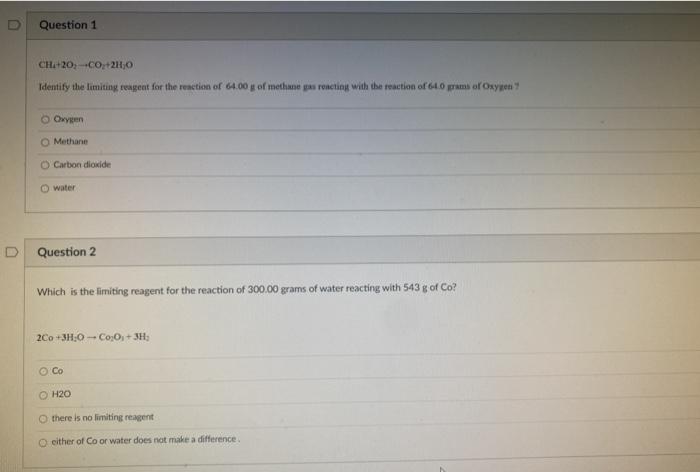
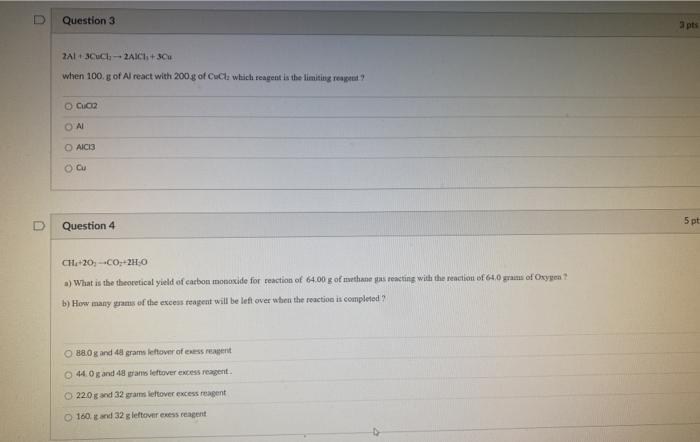
Question 1 CHA+20, +CO +2H30 Identify the limiting reagent for the reaction of 64.00 g of methane pas roncting with the reaction of 61.0 pm or Oxygen Owen Methane Carbon dioxide water Question 2 Which is the limiting reagent for the reaction of 300.00 grams of water reacting with 543 8 of Co? 2Co +3H:0 - Co,O, +3H H2O there is no limiting reagent either of Co or water does not make a difference Question 3 a pts 2A1-3-2AICI.+ 3 when 100. g of Al react with 200 g of Cul which reagent is the limiting real? Cuc AICIS Cu 5 pt Question 4 CH20-C02.0 a) What is the theoretical yield of carbon monoxide for reaction of 64.00 g of methane as reacting with the reaction of 640g of Oxygen b) How many grams of the excess reagent will be left over when the reaction is completed O Box and 48 grams leftover of ressement 44.0 and 48 grams leftover excess reagent 22.0 and 32 grams lietover excess agent 160 and 32 g leftover exess reagent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


