Question: Question 1 Going down a group in the periodic table, atomic size increases because the valence electrons iatetee hae one option from each menu. become
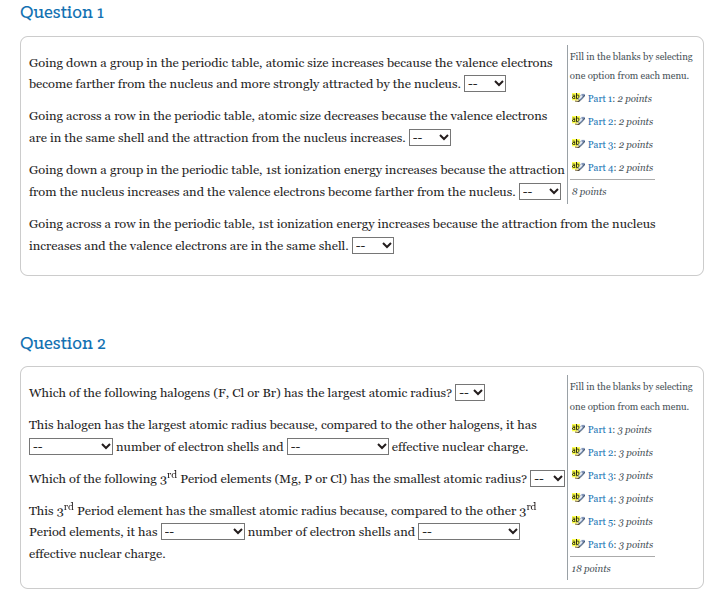 Question 1 Going down a group in the periodic table, atomic size increases because the valence electrons iatetee hae one option from each menu. become farther from the nucleus and more strongly attracted by the nucleus. = al? Part 1: 2 points Going across a row in the periodic table, atomic size decreases because the valence electrons are in the same shell and the attraction from the nucleus increases. al? Part 2: 2 points al? Part 3: 2 points Going down a group in the periodic table, ist ionization energy increases because the attraction a? Part 4: 2 points from the nucleus increases and the valence electrons become farther from the nucleus.
Question 1 Going down a group in the periodic table, atomic size increases because the valence electrons iatetee hae one option from each menu. become farther from the nucleus and more strongly attracted by the nucleus. = al? Part 1: 2 points Going across a row in the periodic table, atomic size decreases because the valence electrons are in the same shell and the attraction from the nucleus increases. al? Part 2: 2 points al? Part 3: 2 points Going down a group in the periodic table, ist ionization energy increases because the attraction a? Part 4: 2 points from the nucleus increases and the valence electrons become farther from the nucleus.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


