Question: QUESTION 1 ( PO 3 , CO 1 , C 2 , C 3 , C 4 ) A feed slurry with a flowrate of
QUESTION
PO CO C C C
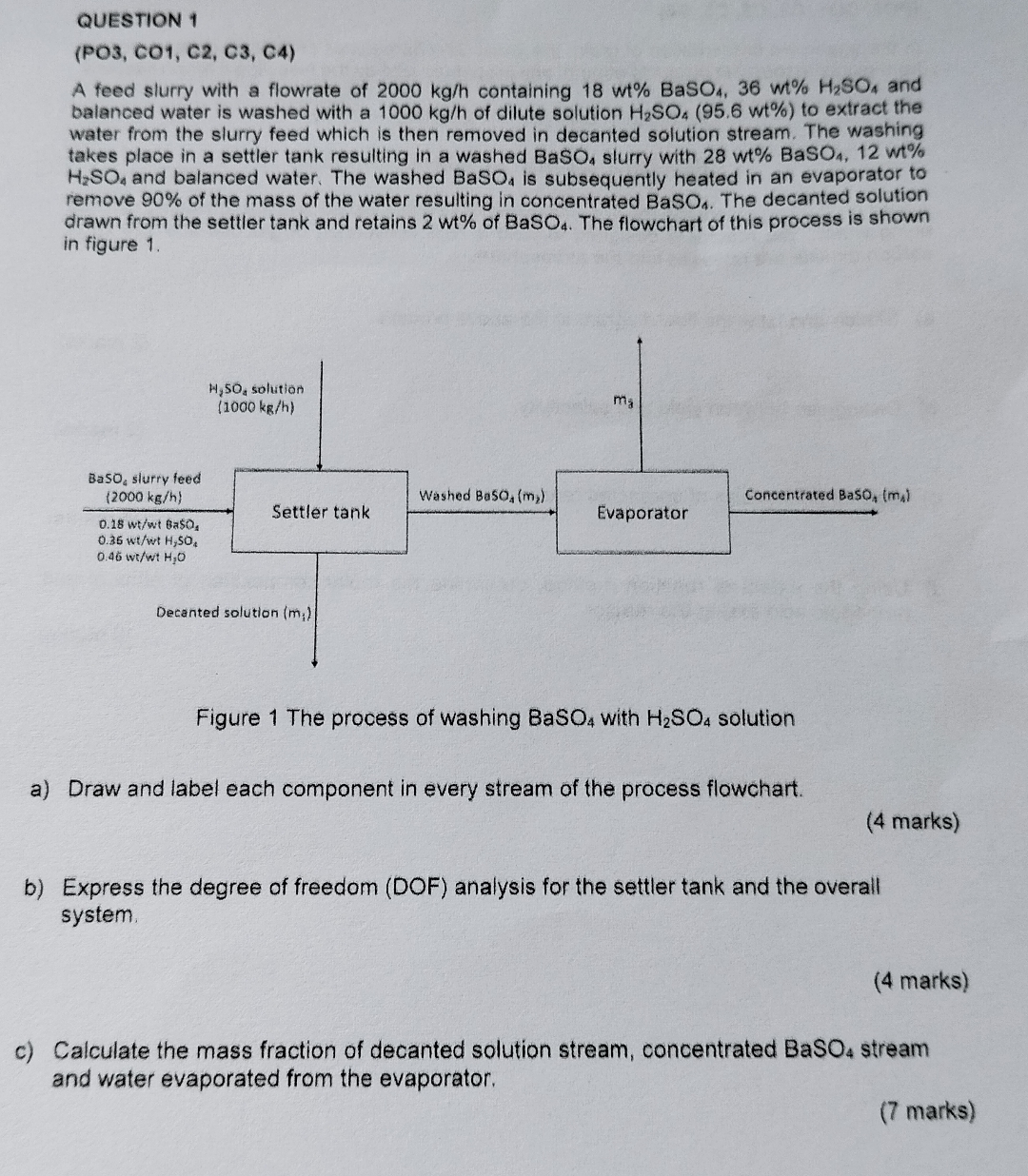
A feed slurry with a flowrate of containing and balanced water is washed with a of dilute solution to extract the water from the slurry feed which is then removed in decanted solution stream. The washing takes place in a settler tank resulting in a washed slurry with wt wt and balanced water. The washed is subsequently heated in an evaporator to remove of the mass of the water resulting in concentrated The decanted solution drawn from the settler tank and retains of The flowchart of this process is shown in figure
Figure The process of washing with solution
a Draw and label each component in every stream of the process flowchart.
marks
b Express the degree of freedom DOF analysis for the settler tank and the overall system.
marks
c Calculate the mass fraction of decanted solution stream, concentrated stream and water evaporated from the evaporator.
marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


