Question: Question: (1 Point) An Electron In A Hydrogen Atom Is In The State |?) = |N=3,1 = 1,J= , M = 1) + V In=2,1
Question: (1 Point) An Electron In A Hydrogen Atom Is In The State |?) = |N=3,1 = 1,J= , M = 1) + V In=2,1 = 0, J = },M=-) Where J And M Are The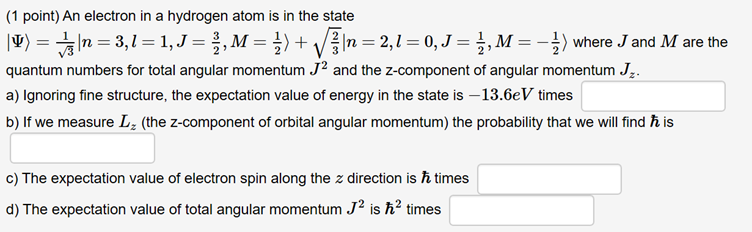
(1 point) An electron in a hydrogen atom is in the state |) = |n = 3,1 = 1, J = , M = ) + } \|n = 2,1 = 0, J = , M = ) where J and M are the quantum numbers for total angular momentum J2 and the z-component of angular momentum Jr. a) Ignoring fine structure, the expectation value of energy in the state is -13.6eV times b) If we measure L (the z-component of orbital angular momentum) the probability that we will find his c) The expectation value of electron spin along the z direction is times d) The expectation value of total angular momentum J is h times
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


