Question: Question 1 question 2 Identify the correct statement(s) regarding thermodynamic versus kinetic control of organic reactions. Higher temperatures and longer reaction times typically favor the
Question 1

question 2
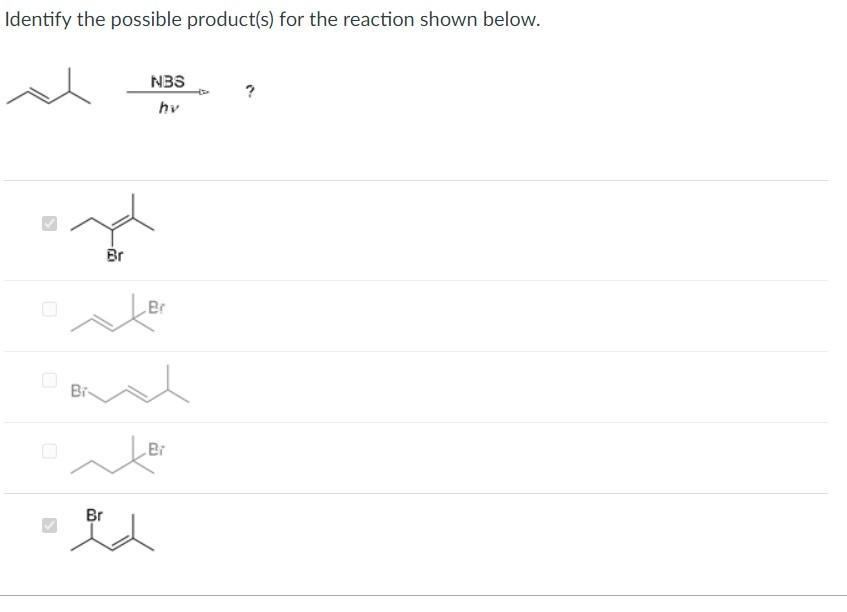
Identify the correct statement(s) regarding thermodynamic versus kinetic control of organic reactions. Higher temperatures and longer reaction times typically favor the thermodynamic product, because the reaction becomes reversible. When a reaction is under kinetic control, the relative amounts of products depend on the relative stabilities of the transition states. Under thermodynamic conditions the major product is the one that is the most stable. The thermodynamic product is always the 1,4 -addition product. The kinetic product is always the 1,2 -addition product. Identify the possible product(s) for the reaction shown below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


