Question: Question 1. R3 Complete the acid-base reaction below by illustrating the loss of a single acidic proton from methylphosphonic acid using the appropriate curved mechanistic
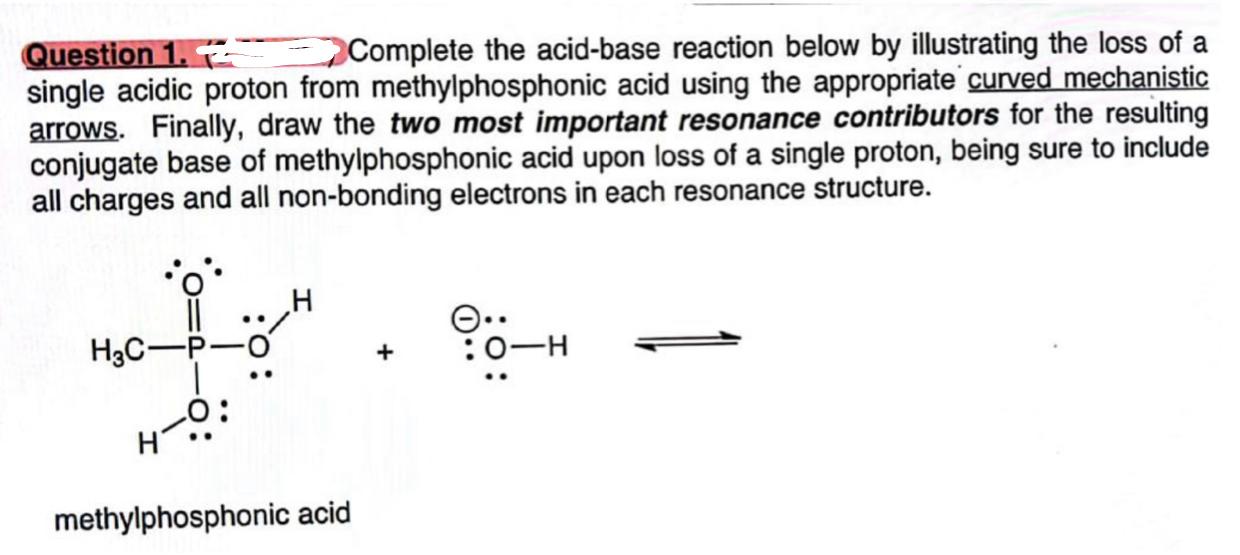
Question 1. R3 Complete the acid-base reaction below by illustrating the loss of a single acidic proton from methylphosphonic acid using the appropriate curved mechanistic arrows. Finally, draw the two most important resonance contributors for the resulting conjugate base of methylphosphonic acid upon loss of a single proton, being sure to include all charges and all non-bonding electrons in each resonance structure. +OH methylphosphonic acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


