Question: The questions on the quiz will reference the following reaction: When siver ntrate and sodium iodide are mixed in aqueous solution, they participate in
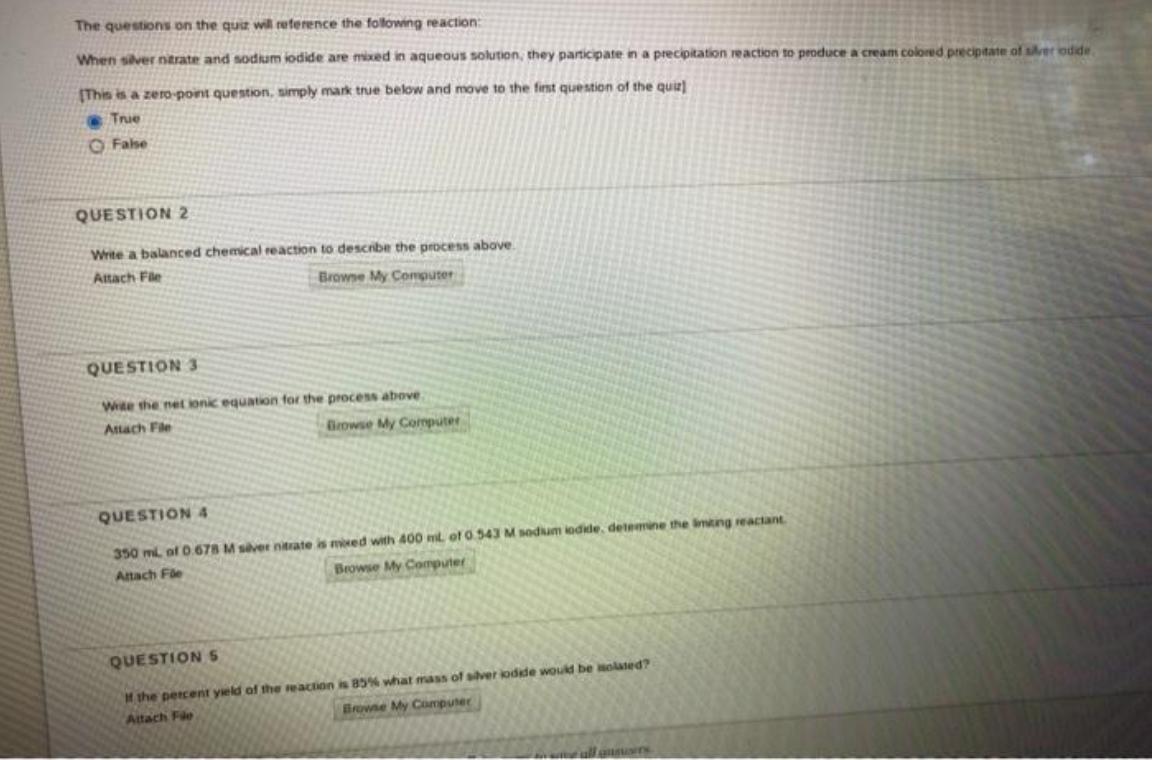
The questions on the quiz will reference the following reaction: When siver ntrate and sodium iodide are mixed in aqueous solution, they participate in a precipitation reaction to produce a cream colored precipitate of srer odde (This is a zero-point question, simply mark true below and move to the fint question of the quie) True O False QUESTION 2 Write a balanced chemical reaction to describe the pocess above. Attach Fle Browse My Computer QUESTION 3 Wite the net ionic equation for the process above Attach Fle Browse My Coputer QUESTION 4 350 ml of .678 M svet nitrate is moed with 400 mt of 0.543 M sodium iodide, detemine the imting reactant Attach Fle Browse My Computer QUESTION 5 Mthe percent yeld of the reaction is 85% what mass of silver kodde would be Holated? Browne My Computer Attach Fle
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


