Question: Question 1 (This is a multiple response question. There may be one or more correct statements. You will gain credits for yo In the phase
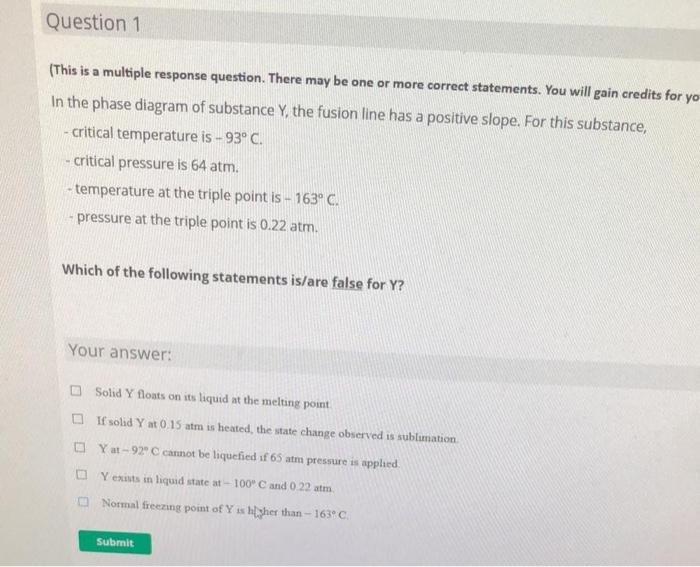
Question 1 (This is a multiple response question. There may be one or more correct statements. You will gain credits for yo In the phase diagram of substance Y, the fusion line has a positive slope. For this substance, critical temperature is - 93 C. critical pressure is 64 atm. - temperature at the triple point is - 163C. - pressure at the triple point is 0.22 atm. Which of the following statements is/are false for Y? Your answer: Solid Y floats on its liquid at the melting point If solid Y at 0.15 atm is heated, the state change observed is sublimation Y at-92 cannot be liquefied if 65 atm pressure is applied Yexists in liquid state at - 100 C and 0 22 atm Normal freezing point of Y is higher than - 163C. Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


