Question: Question 1. This problem is a (#throwback) to material from earlier in the semester. Consider the imple series reaction mechanism taking place in a constant-volume
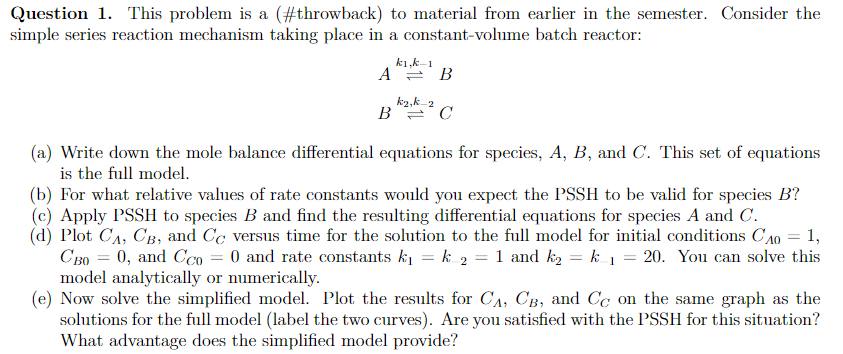
Question 1. This problem is a (\#throwback) to material from earlier in the semester. Consider the imple series reaction mechanism taking place in a constant-volume batch reactor: Ak1,k1BBk2,k2C (a) Write down the mole balance differential equations for species, A,B, and C. This set of equations is the full model. (b) For what relative values of rate constants would you expect the PSSH to be valid for species B ? (c) Apply PSSH to species B and find the resulting differential equations for species A and C. (d) Plot CA,CB, and CC versus time for the solution to the full model for initial conditions CA0=1, CB0=0, and CC0=0 and rate constants k1=k2=1 and k2=k1=20. You can solve this model analytically or numerically. (e) Now solve the simplified model. Plot the results for CA,CB, and CC on the same graph as the solutions for the full model (label the two curves). Are you satisfied with the PSSH for this situation? What advantage does the simplified model provide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


