Question: QUESTION 10 a. Consider the electrochemical reaction given below. A13+ + AU A+ A+ Write the separate oxidation and reduction half-equations for this reaction. Oxidation
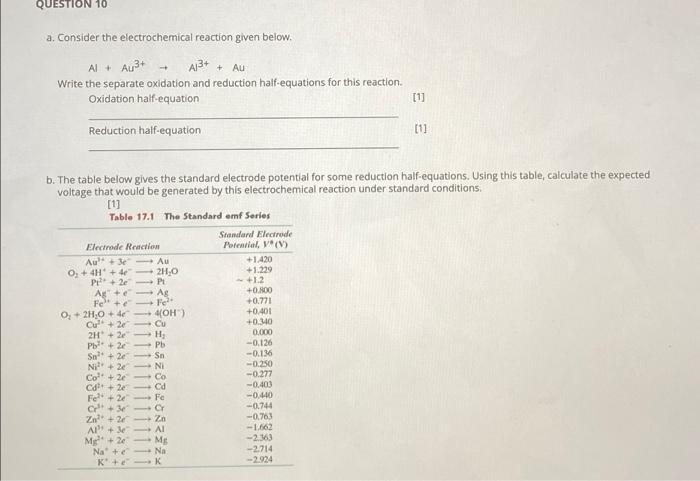
QUESTION 10 a. Consider the electrochemical reaction given below. A13+ + AU A+ A+ Write the separate oxidation and reduction half-equations for this reaction. Oxidation half-equation [1] Reduction half-equation [11 0 Te b. The table below gives the standard electrode potential for some reduction half-equations. Using this table, calculate the expected voltage that would be generated by this electrochemical reaction under standard conditions, Table 17.1 The Standard emf Series Standard Electrode Electrode Reaction Polnilal, V (1) Au +3 Au +1.420 O, + 4H+ 4 2H2O +1.229 P + 2 PE Ag+ Ag +0.NO Fe + +0,721 O, + 2H2O + 4 4OH) +0.401 Cul + 20 Cu +0.340 2H + 2H 0.000 P + 2 Pb -0,126 Sn + 2-Sn -0.136 Nil' + 2 NI -0.250 Col + 2 Co -0.277 C+2 ca -0.403 Fe2+ + 2 Fe -0.440 Cr +34 CY -0.744 Zn +20 -0.763 AN de Al -1.662 M+2 ME -263 Na + c Na -2.714 K+K -2.924
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


