Question: Question 10 The experimental enthalpy curve of a binary solution of components 1 and 2 at constant T and P is given by: h=100x1+600x2+x1x2(10x1+20x2)J/mol i)
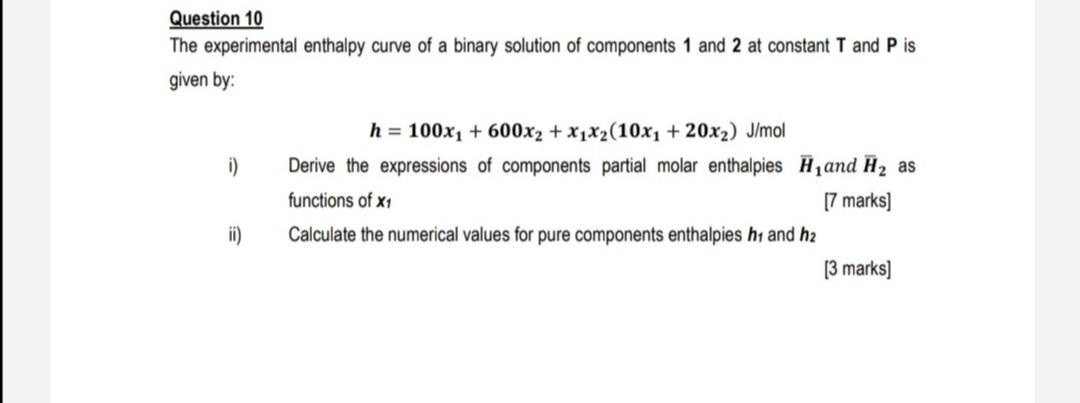
Question 10 The experimental enthalpy curve of a binary solution of components 1 and 2 at constant T and P is given by: h=100x1+600x2+x1x2(10x1+20x2)J/mol i) Derive the expressions of components partial molar enthalpies H1 and H2 as functions of x1 [7 marks] ii) Calculate the numerical values for pure components enthalpies h1 and h2 [3 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


