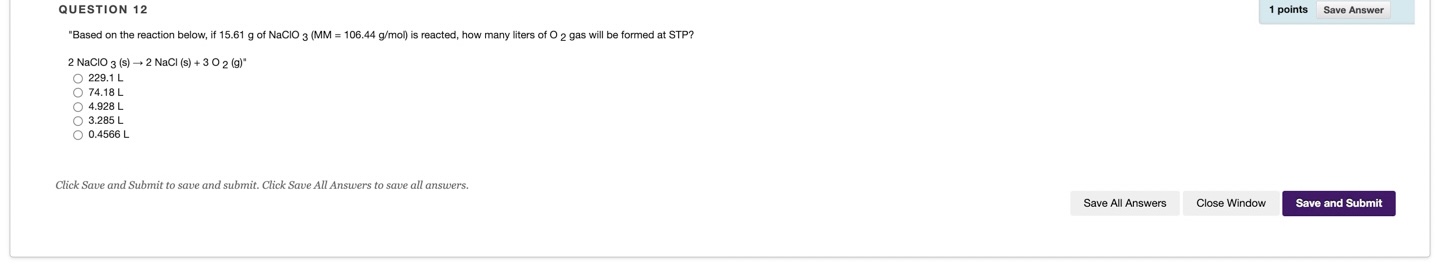
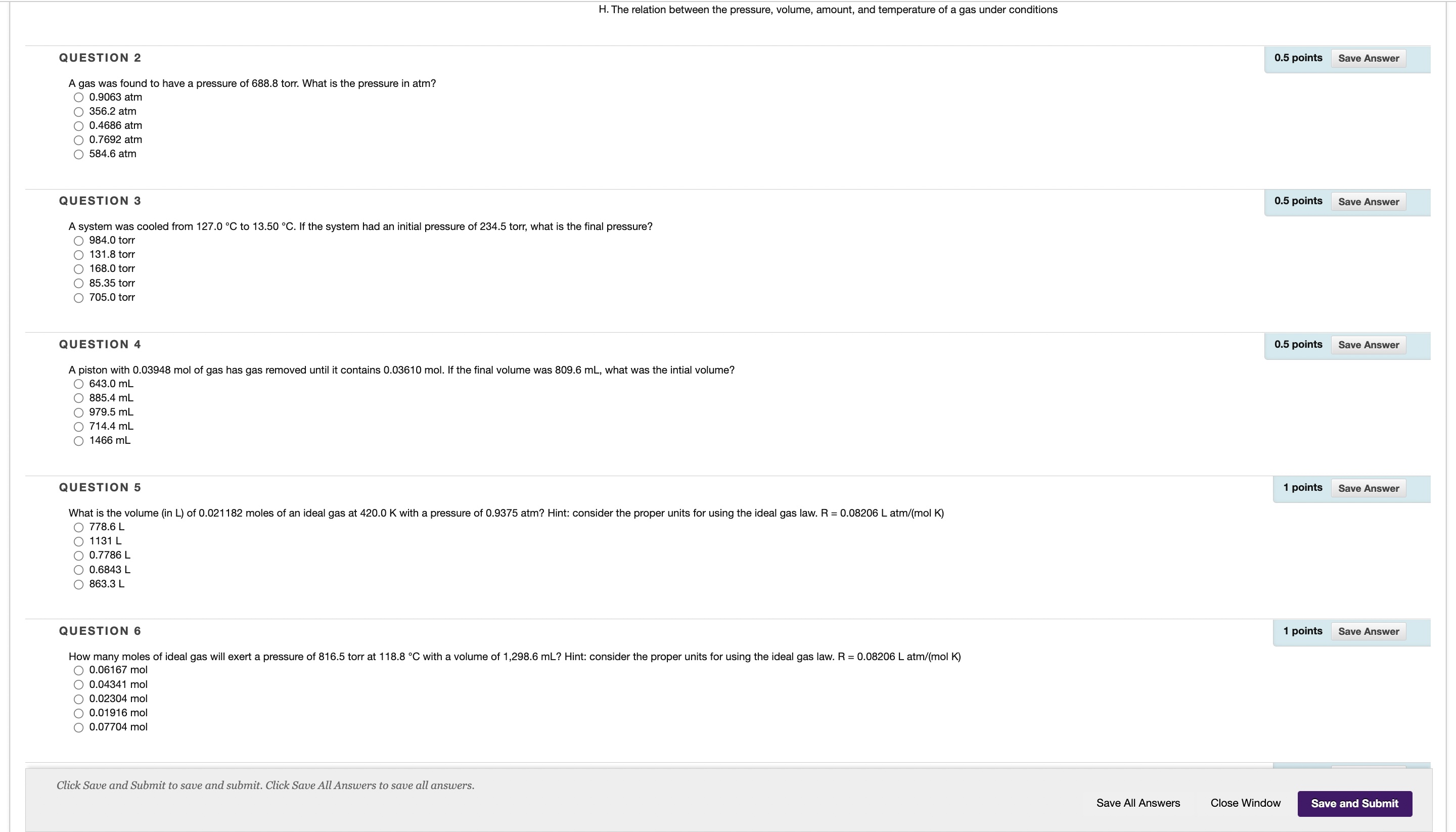
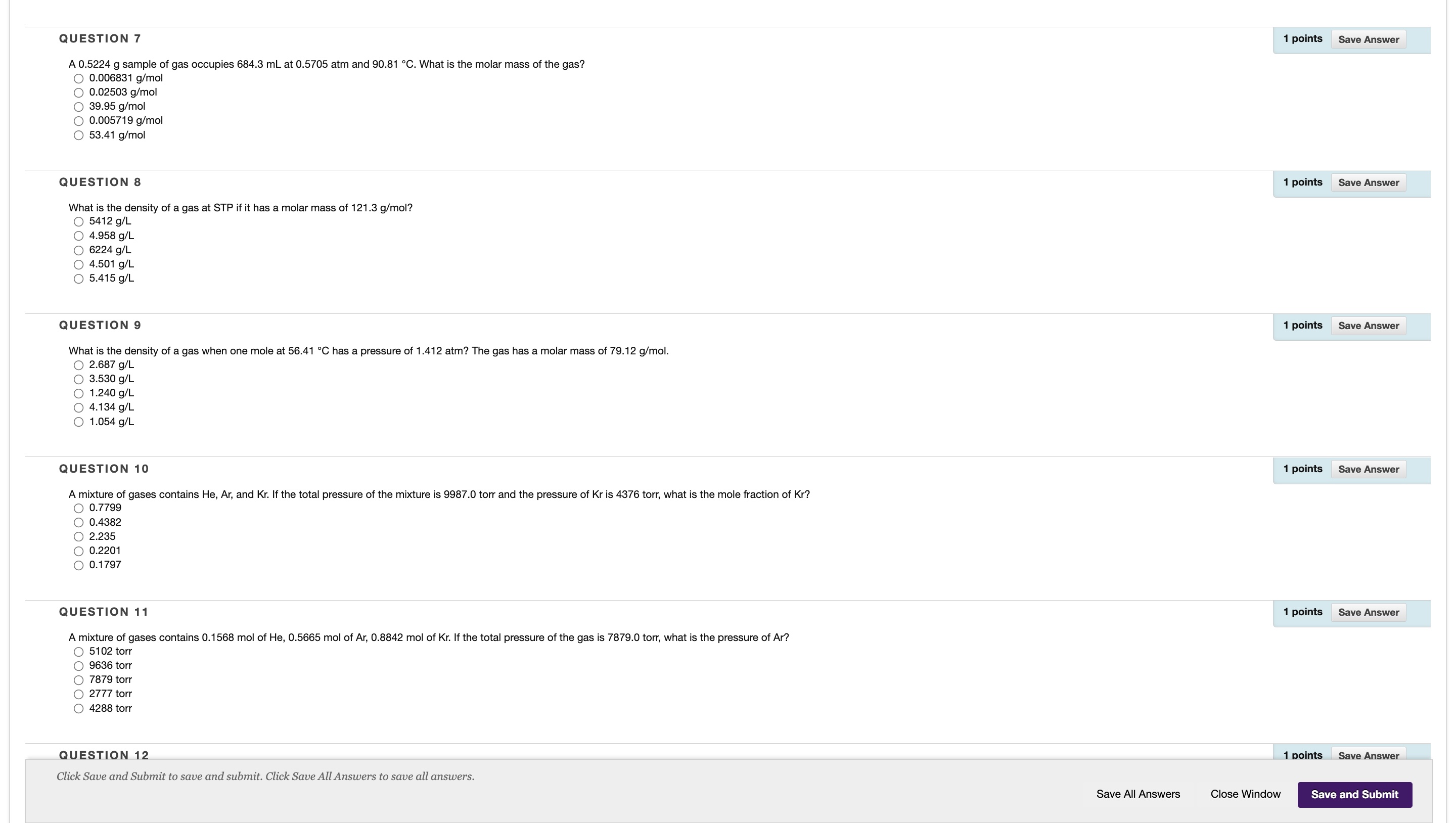
Question: QUESTION 12 1 points Save Answer Based on the reaction below, if 15.61 g of NaCIO 3 (MM = 106.44 g/mol) is reacted, how many
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


