Question: Question 12 Using the phase diagram, given the pressure and temperature tell whether H20 is liquid, solid or gas. Type L for liquid, S for
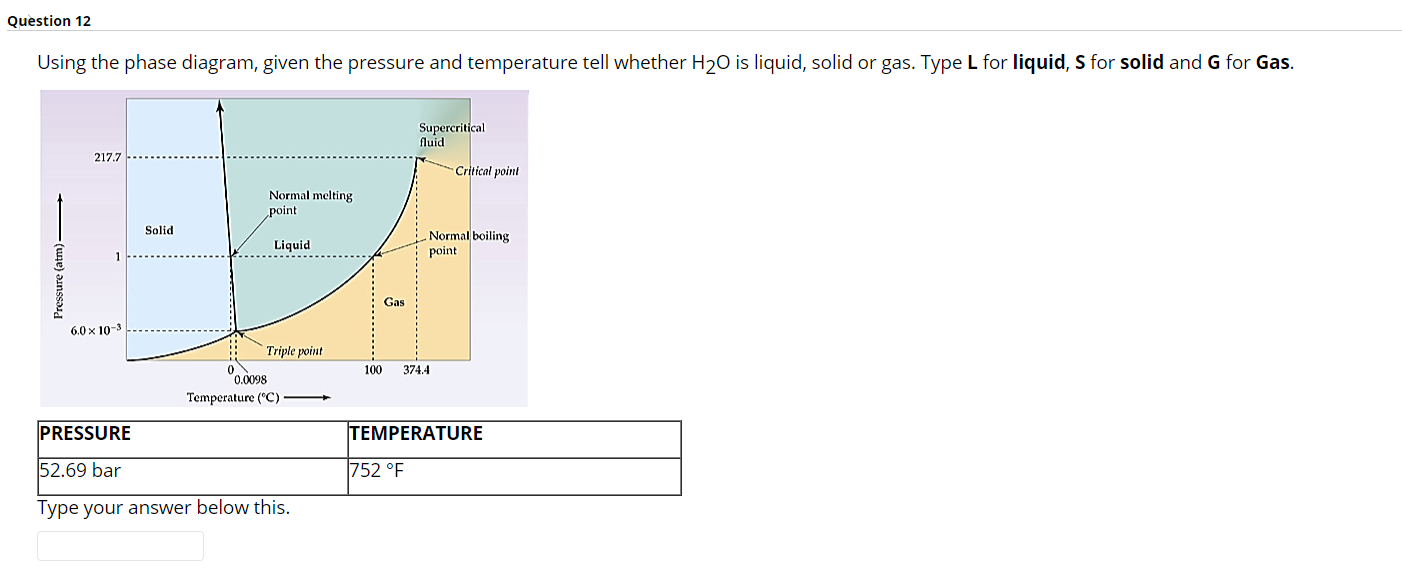
Question 12 Using the phase diagram, given the pressure and temperature tell whether H20 is liquid, solid or gas. Type L for liquid, S for solid and G for Gas. Supercritical fluid 217.7 Critical point Normal melting point Solid Liquid Normal boiling point Pressure (atm) Gas 6.0 x 10-3 Triple point 100 374.4 0.0098 Temperature (C) PRESSURE TEMPERATURE 52.69 bar 752 F Type your answer below this
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


