Question: Question 13 of15 - /1 '5 View Policies Current Attempt in Progress Find the energy (in joules) of the photon that is emitted when the
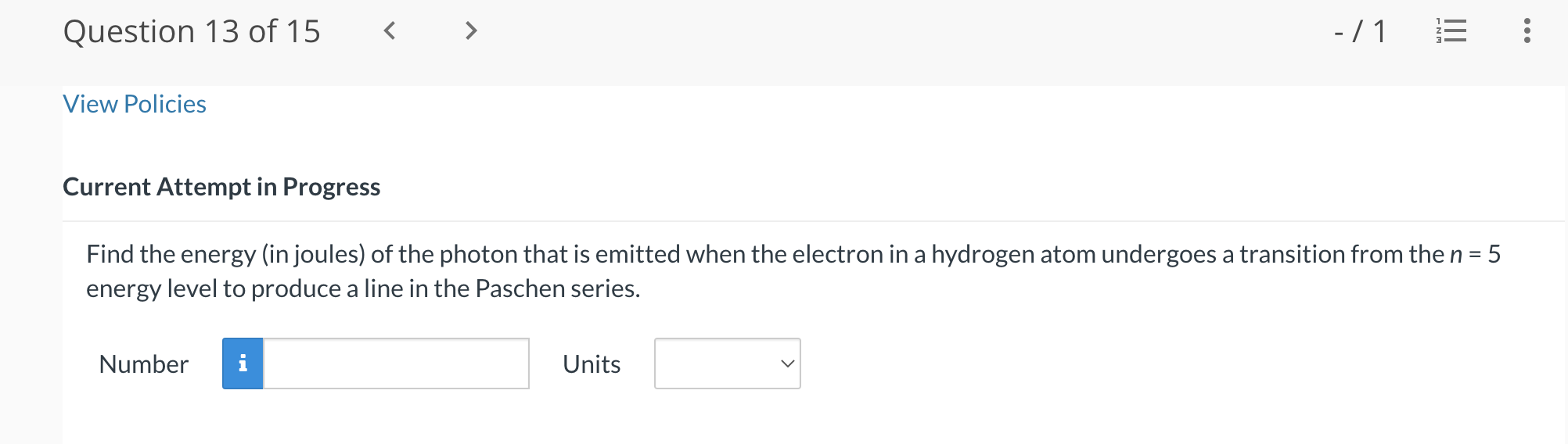
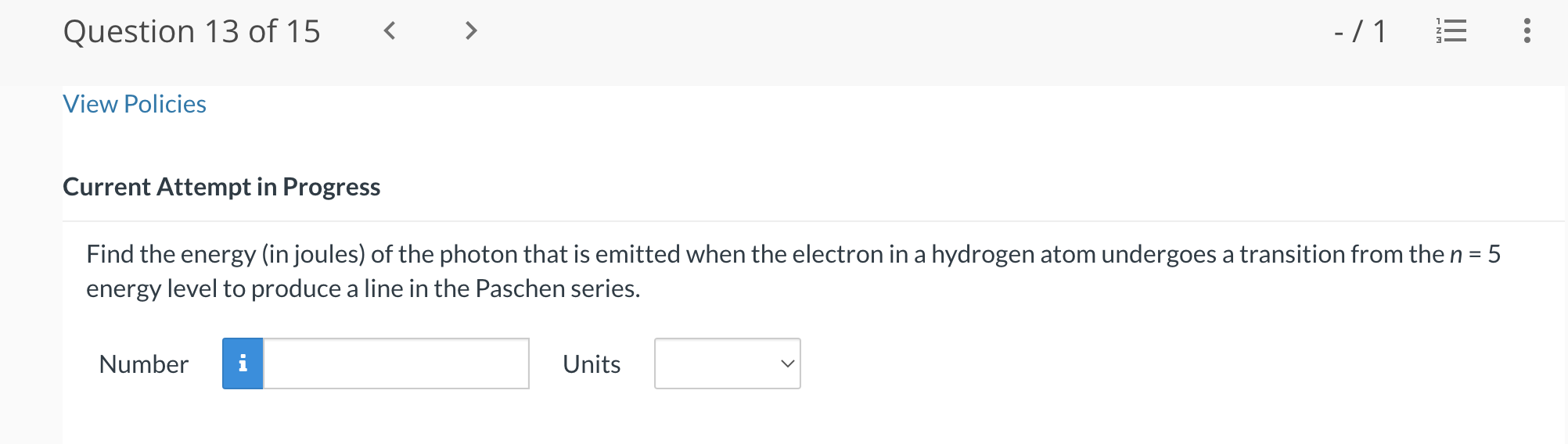
Question 13 of15 - /1 '5 View Policies Current Attempt in Progress Find the energy (in joules) of the photon that is emitted when the electron in a hydrogen atom undergoes a transition from the n = 5 energy level to produce a line in the Paschen series. Number Units V
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


