Question: Which statement is incorrect? O a. Once an electron has been removed from a neutral atom, the second electron is usually easier to remove.
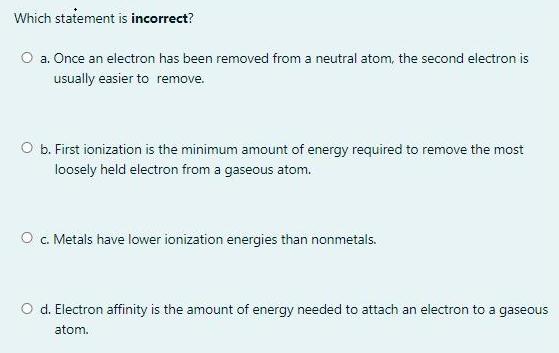

Which statement is incorrect? O a. Once an electron has been removed from a neutral atom, the second electron is usually easier to remove. O b. First ionization is the minimum amount of energy required to remove the most loosely held electron from a gaseous atom. O . Metals have lower ionization energies than nonmetals. O d. Electron affinity is the amount of energy needed to attach an electron to a gaseous atom. Question 12 An element that ends in ns? in its ground state electron configuration can be Not yet answered Marked out of O a. transition element 1.25 P Flag question O b. alkali metal O c. representative element O d. halogen
Step by Step Solution
3.41 Rating (173 Votes )
There are 3 Steps involved in it
Usually when an electron is removed out of an atom it makes further removal of ... View full answer

Get step-by-step solutions from verified subject matter experts


