Question: Question 18.d of 36 Submit A solution is made by dissolving 29.3 g of Ba(NO2)2 in 500.0 mL of water. What is the value of
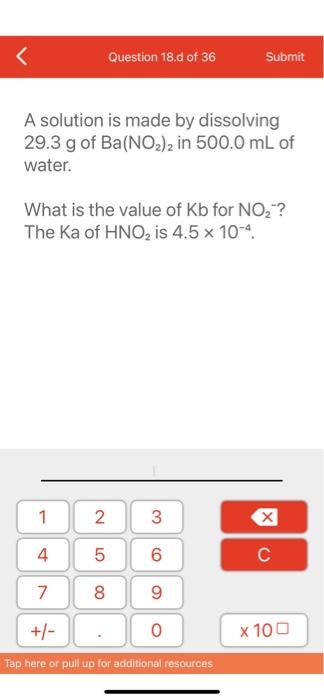
Question 18.d of 36 Submit A solution is made by dissolving 29.3 g of Ba(NO2)2 in 500.0 mL of water. What is the value of Kb for NO2? The Ka of HNO2 is 4.5 x 10-4 1 2 3 4 st 5 5 6 7 8 9 +/- 0 x 100 Tap here or pull up for additional resources
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


