Question: Question 2 (18 marks) A spent isopropyl ether with minor amount of acetic acid (2.5wt%) is used to extract more acetic acid from a water

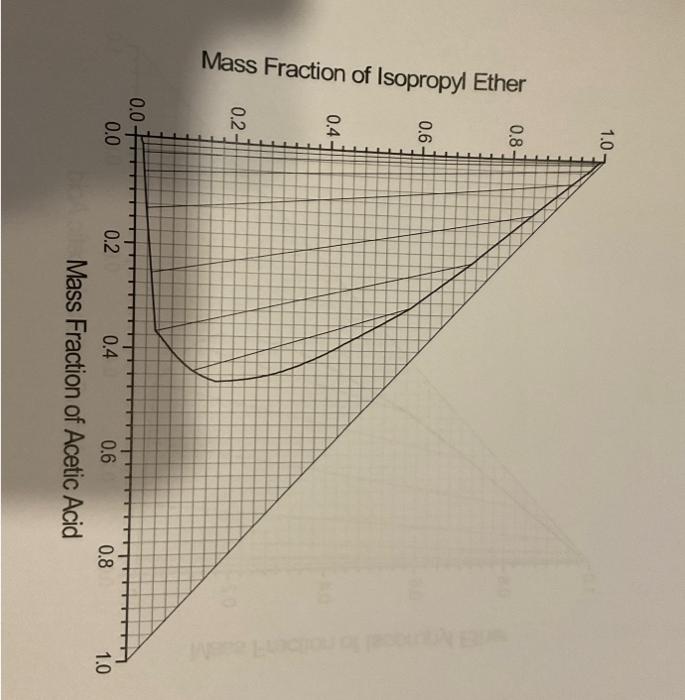
Question 2 (18 marks) A spent isopropyl ether with minor amount of acetic acid (2.5wt%) is used to extract more acetic acid from a water stream (300kg/h) in a countercurrent multistage system. The water stream contains 35wt% acetic acid. The exit acetic acid concentration in the water stream is supposed to be 10wt%. Show your workings on the equilibrium diagram for isopropyl ether and acetic acid on Page 5 and Page 6 and return the whole question paper with the answer booklet for submission. 2.1 Using equilibrium diagram on Page 5, determine the compositions for the mixture and the ether extract V1min at the minimum solvent rate VN+1min. (6 marks) 2.2 Using equilibrium diagram on Page 6, Calculate the number of stages required to achieve the desired exiting concentration of acetic acid at VN+1=1.5VN+1min. (12 marks) Mass Fraction nf Question 2 (18 marks) A spent isopropyl ether with minor amount of acetic acid (2.5wt%) is used to extract more acetic acid from a water stream (300kg/h) in a countercurrent multistage system. The water stream contains 35wt% acetic acid. The exit acetic acid concentration in the water stream is supposed to be 10wt%. Show your workings on the equilibrium diagram for isopropyl ether and acetic acid on Page 5 and Page 6 and return the whole question paper with the answer booklet for submission. 2.1 Using equilibrium diagram on Page 5, determine the compositions for the mixture and the ether extract V1min at the minimum solvent rate VN+1min. (6 marks) 2.2 Using equilibrium diagram on Page 6, Calculate the number of stages required to achieve the desired exiting concentration of acetic acid at VN+1=1.5VN+1min. (12 marks) Mass Fraction nf
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


