Question: Question 2 2) 8 Mn04 + 5 H25 + 19 H+ -> 8 Mn2+ + 5 HSO4 + 12 H20 identify as the oxidizing agent,
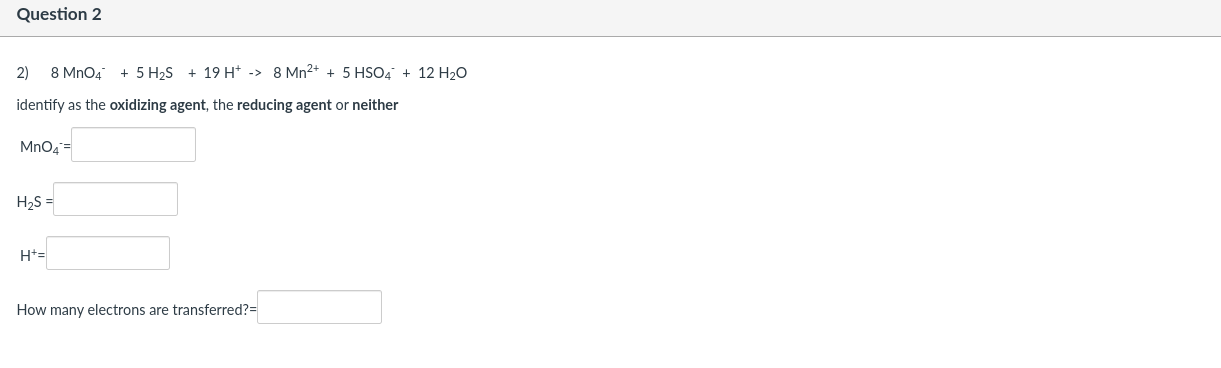
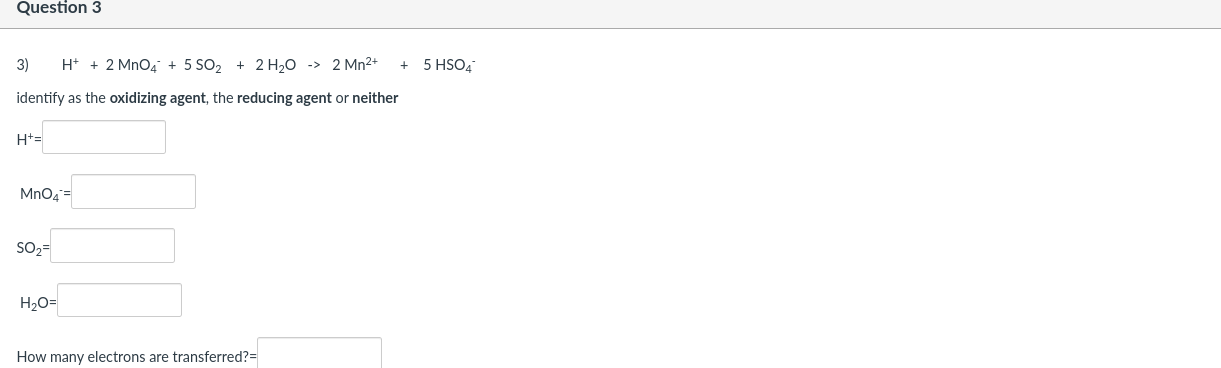
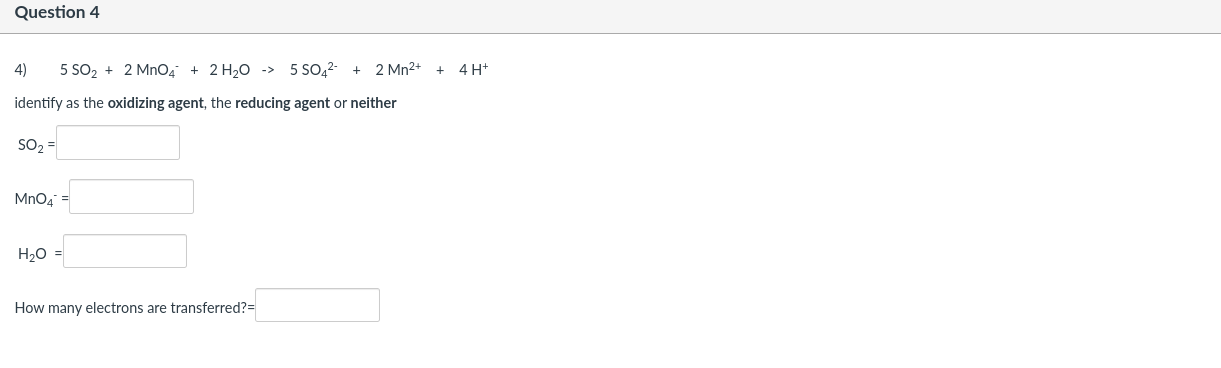
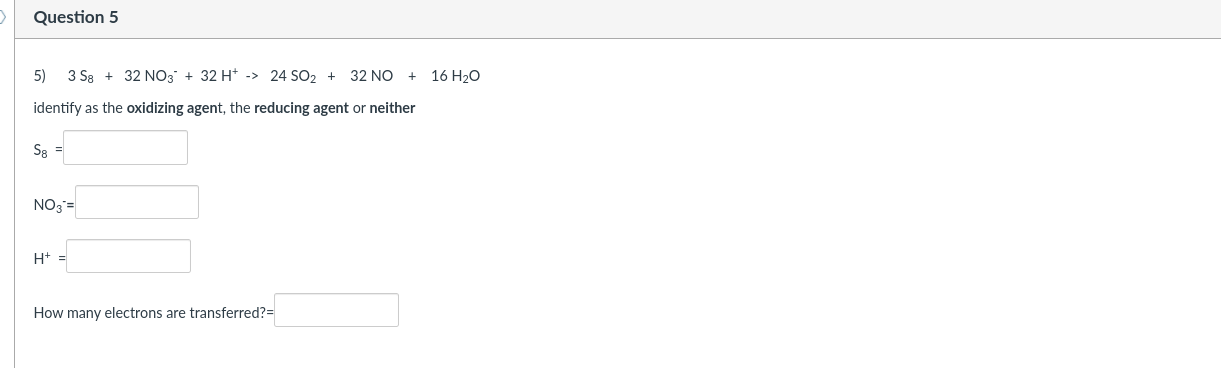
Question 2 2) 8 Mn04 + 5 H25 + 19 H+ -> 8 Mn2+ + 5 HSO4 + 12 H20 identify as the oxidizing agent, the reducing agent or neither MnO4 = H2S = H= How many electrons are transferred? Question 3 5 HSO4 3) H+ + 2 MnO4 + 5 SO2 + 2 H2O -> 2 Mn2+ identify as the oxidizing agent, the reducing agent or neither H+= Mn04 SO2- H20- How many electrons are transferred? Question 4 4) 5 SO2 + 2 MnO4 + 2 H2O -> 5 SO42- + 2 Mn2+ + 4H+ identify as the oxidizing agent, the reducing agent or neither SO2- Mn04 H20 = How many electrons are transferred? Question 5 5) 358 + 32 NO3 + 32 H+ -> 24 SO2 + 32 NO + 16 H20 identify as the oxidizing agent, the reducing agent or neither S8 = NO3 = H+ = How many electrons are transferred
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


