Question: Question 2: (20 marks) In the Ostwald process for oxidizing ammonia, a variety of products is possible N2, NO, NO, and NO, depending on the
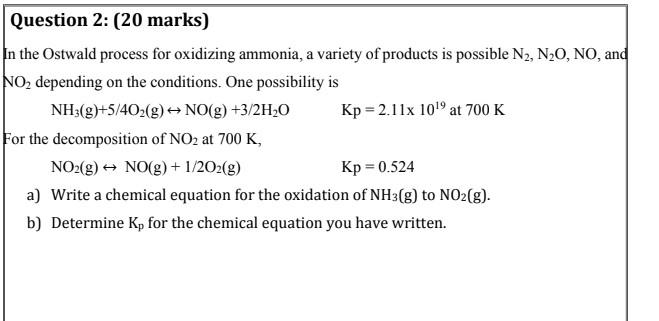
Question 2: (20 marks) In the Ostwald process for oxidizing ammonia, a variety of products is possible N2, NO, NO, and NO, depending on the conditions. One possibility is NH3(g)+5/402(g) NO(g) +3/2H20 Kp = 2.11x 109 at 700 K For the decomposition of NO2 at 700 K, NO2(g) + NO(g) + 1/2O2(g) Kp = 0.524 a) Write a chemical equation for the oxidation of NH3(g) to NO2(g). b) Determine K, for the chemical equation you have written
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


