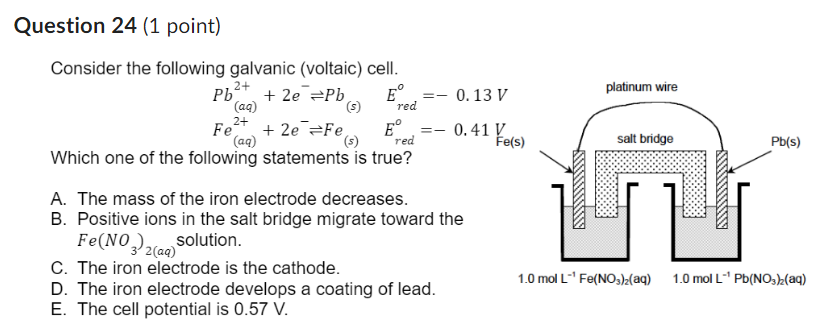
Question: Question 2 4 ( 1 point ) Consider the following galvanic ( voltaic ) cell. P b ( a q ) 2 + + 2
Question point
Consider the following galvanic voltaic cell.
Which one of the following statements is true?
A The mass of the iron electrode decreases.
B Positive ions in the salt bridge migrate toward the
solution.
C The iron electrode is the cathode.
D The iron electrode develops a coating of lead.
E The cell potential is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


