Question: Question 2 ( 5 5 points ) : This question has THREE parts. Answer all parts. Show calculations and provide logical explanations wherever required. a
Question points: This question has THREE parts. Answer all
parts. Show calculations and provide logical explanations wherever
required.
a Which statement of the nd Law of Thermodynamics is applicable to the
following:
i Although all work can be converted completely to heat, heat
cannot be completely and continuously converted into work.
ii Heat flows spontaneously from high to low temperature, but not
conversely.
iii It is impossible to construct a cyclically operating device for which
the sole effect is the extraction of heat from a single thermal
reservoir and the creation of an equivalent amount of work.
iv No power cycle can have a thermal efficiency of
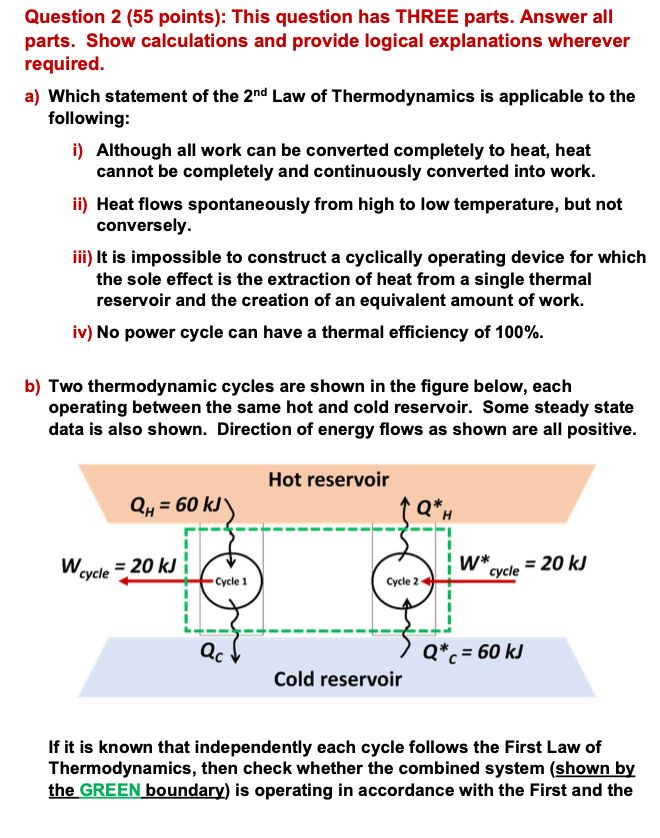
b Two thermodynamic cycles are shown in the figure below, each
operating between the same hot and cold reservoir. Some steady state
data is also shown. Direction of energy flows as shown are all positive.
If it is known that independently each cycle follows the First Law of
Thermodynamics, then check whether the combined system shown by
the GREEN boundary is operating in accordance with the First and the
Second Laws of Thermodynamics. Validation of the Second Law is to be
achieved through the validation of the Clausius andor the KelvinPlanck
statements.
c If in addition to the data provided in part b the temperatures of the hot
and the cold reservoirs are given as @C and @C respectively,
then find whether it is possible to independently operate the Cycle If
yes, then is it a reversible cycle or an irreversible cycle? Second Laws of Thermodynamics. Validation of the Second Law is to be achieved through the validation of the Clausius andor the KelvinPlanck statements.
c If in addition to the data provided in part b the temperatures of the hot and the cold reservoirs are given as circmathrmC and circmathrmC respectively, then find whether it is possible to independently operate the Cycle If yes, then is it a reversible cycle or an irreversible cycle?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


