Question: QUESTION -2: In an industrial process, a two component system: Chloroform (Component 1) and Cyclohexane (Component 2), is used. The system components follow Raoult's Law.
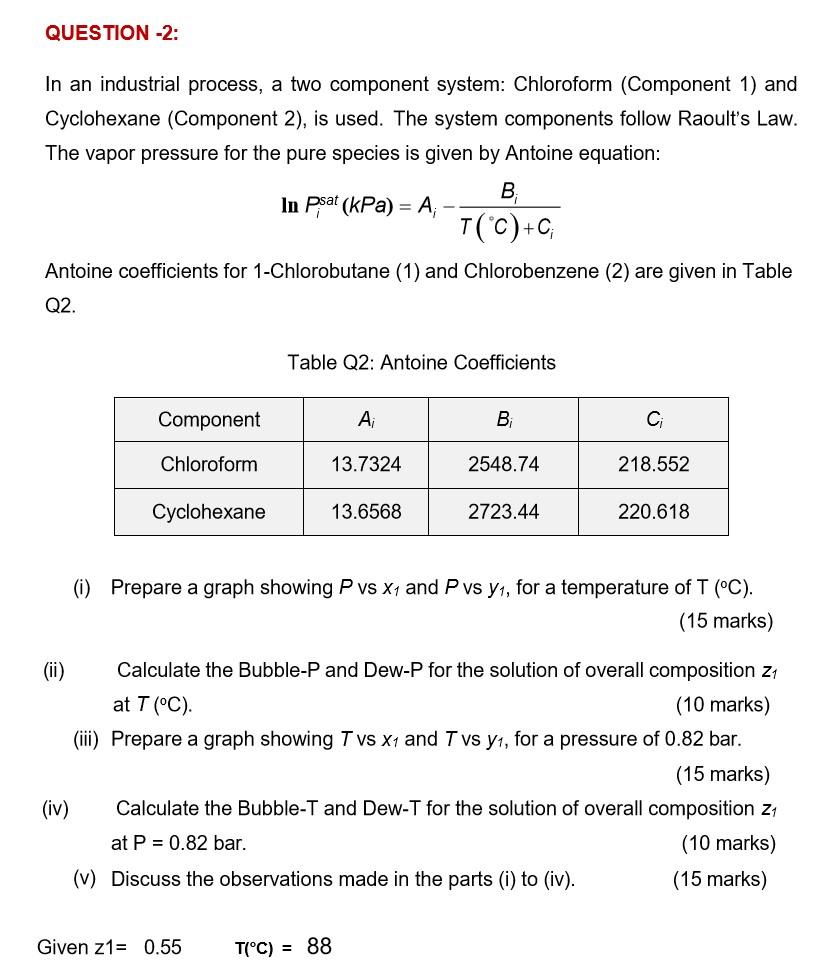
QUESTION -2: In an industrial process, a two component system: Chloroform (Component 1) and Cyclohexane (Component 2), is used. The system components follow Raoult's Law. The vapor pressure for the pure species is given by Antoine equation: . In psat (kPa) = A; T(C)+C; Antoine coefficients for 1-Chlorobutane (1) and Chlorobenzene (2) are given in Table = Q2. Table Q2: Antoine Coefficients Component , C Chloroform 13.7324 2548.74 218.552 Cyclohexane 13.6568 2723.44 220.618 (1) Prepare a graph showing P vs X1 and P vs y, for a temperature of T (C). (15 marks) (ii) Calculate the Bubble-P and Dew-P for the solution of overall composition 21 at T (C). (10 marks) (iii) Prepare a graph showing T vs X1 and T vs y1, for a pressure of 0.82 bar. (15 marks) (iv) Calculate the Bubble-T and Dew-T for the solution of overall composition 21 at P = 0.82 bar. (10 marks) (v) Discuss the observations made in the parts (i) to (iv). (15 marks) Given z1= 0.55 T(C) = 88
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


