Question: Question 2 Several different electrochemical cells can be constructed using the materials listed below. 1.0M solutions of AI(NO3), Fe(NO3)2 and Cu(NO3)2 Metal strips of Al,

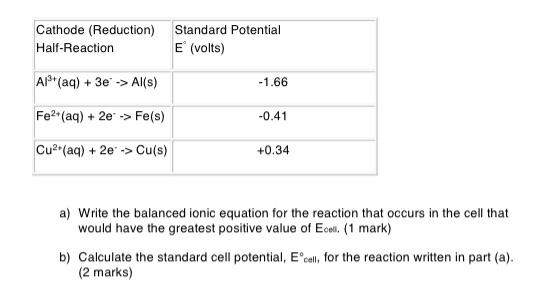
Question 2 Several different electrochemical cells can be constructed using the materials listed below. 1.0M solutions of AI(NO3), Fe(NO3)2 and Cu(NO3)2 Metal strips of Al, Cu and Fe Salt bridge material and solution Cathode (Reduction) Half-Reaction Standard Potential E (volts) AP(aq) + 3e -> Al(s) -1.66 Fe2(aq) + 2e > Fe(s) -0.41 Cu2(aq) + 2e -> Cu(s) +0.34 a) Write the balanced ionic equation for the reaction that occurs in the cell that would have the greatest positive value of Ecell. (1 mark) b) Calculate the standard cell potential, Ecell, for the reaction written in part (a). (2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


