Question: Question 2 The diagram provided illustrates a process for the production of solid potassium chloride ( K C l ) flakes, which are commonly used
Question
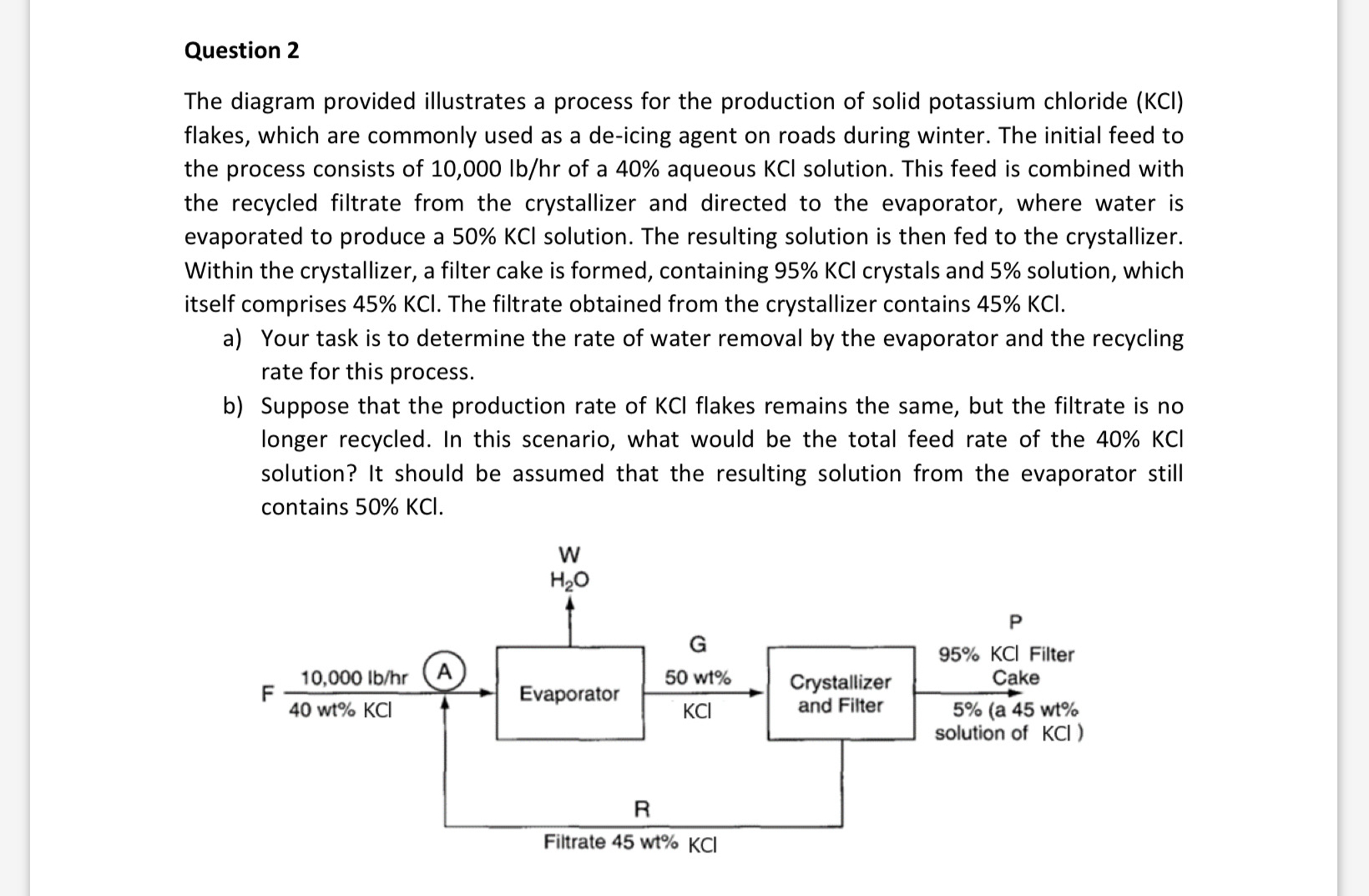
The diagram provided illustrates a process for the production of solid potassium chloride flakes, which are commonly used as a deicing agent on roads during winter. The initial feed to the process consists of of a aqueous solution. This feed is combined with the recycled filtrate from the crystallizer and directed to the evaporator, where water is evaporated to produce a solution. The resulting solution is then fed to the crystallizer. Within the crystallizer, a filter cake is formed, containing crystals and solution, which itself comprises The filtrate obtained from the crystallizer contains
a Your task is to determine the rate of water removal by the evaporator and the recycling rate for this process.
b Suppose that the production rate of flakes remains the same, but the filtrate is no longer recycled. In this scenario, what would be the total feed rate of the solution? It should be assumed that the resulting solution from the evaporator still contains
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


