Question: Question 21 (1 point) Convert 16.81 .C to K. Report to at least 2 decimal places. Your Answer: Answer Question 22 (1 point) Convert 145.57
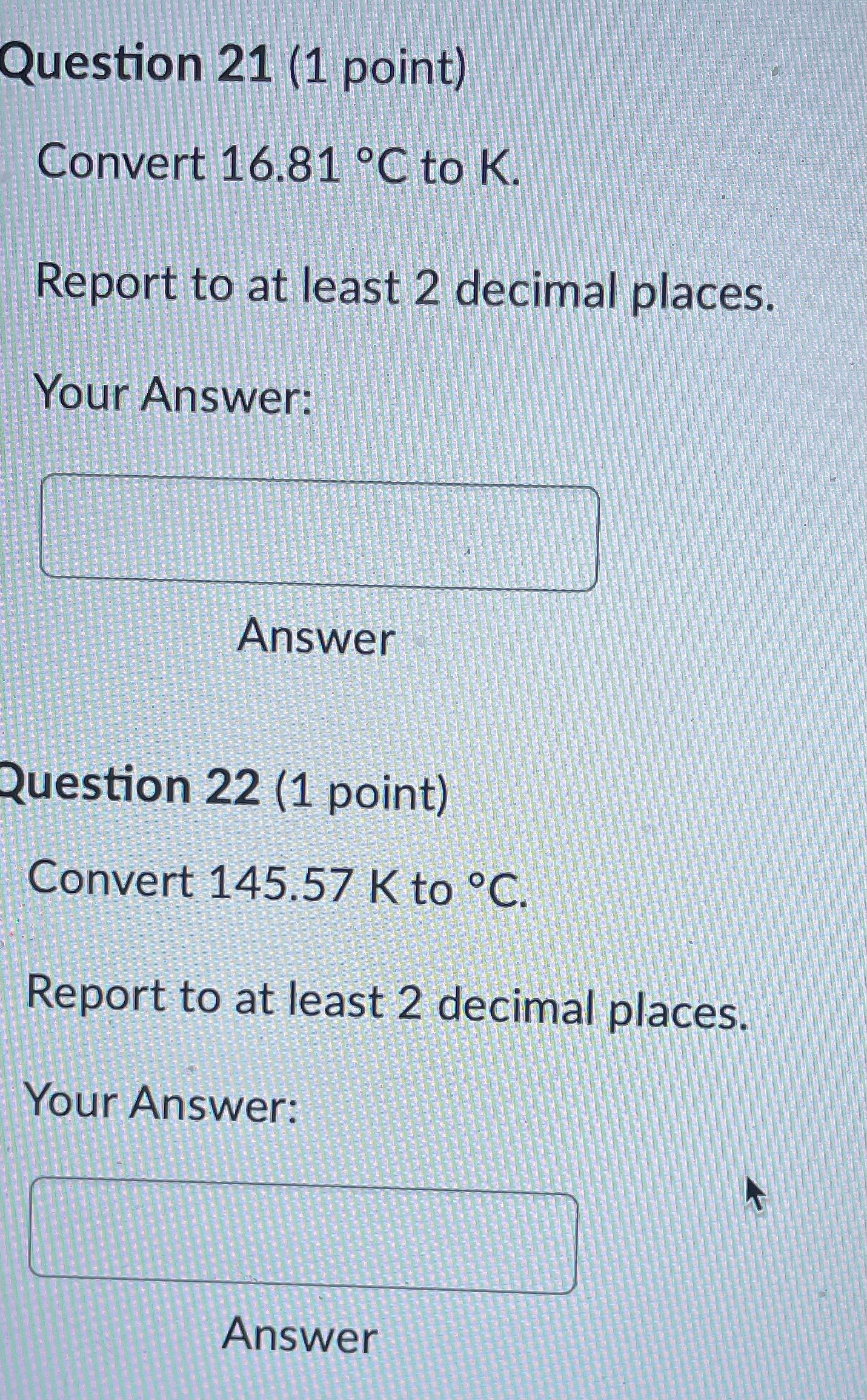
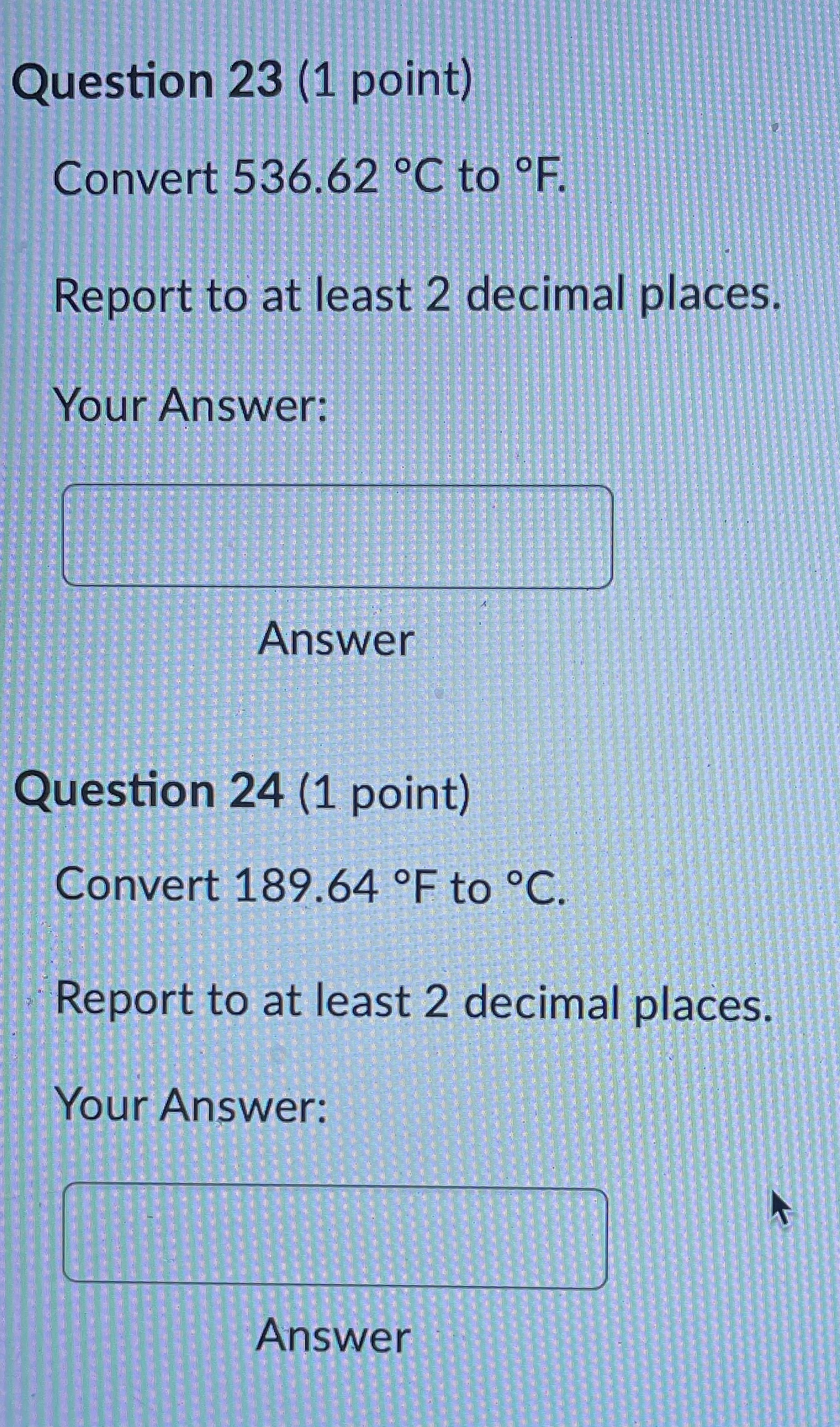

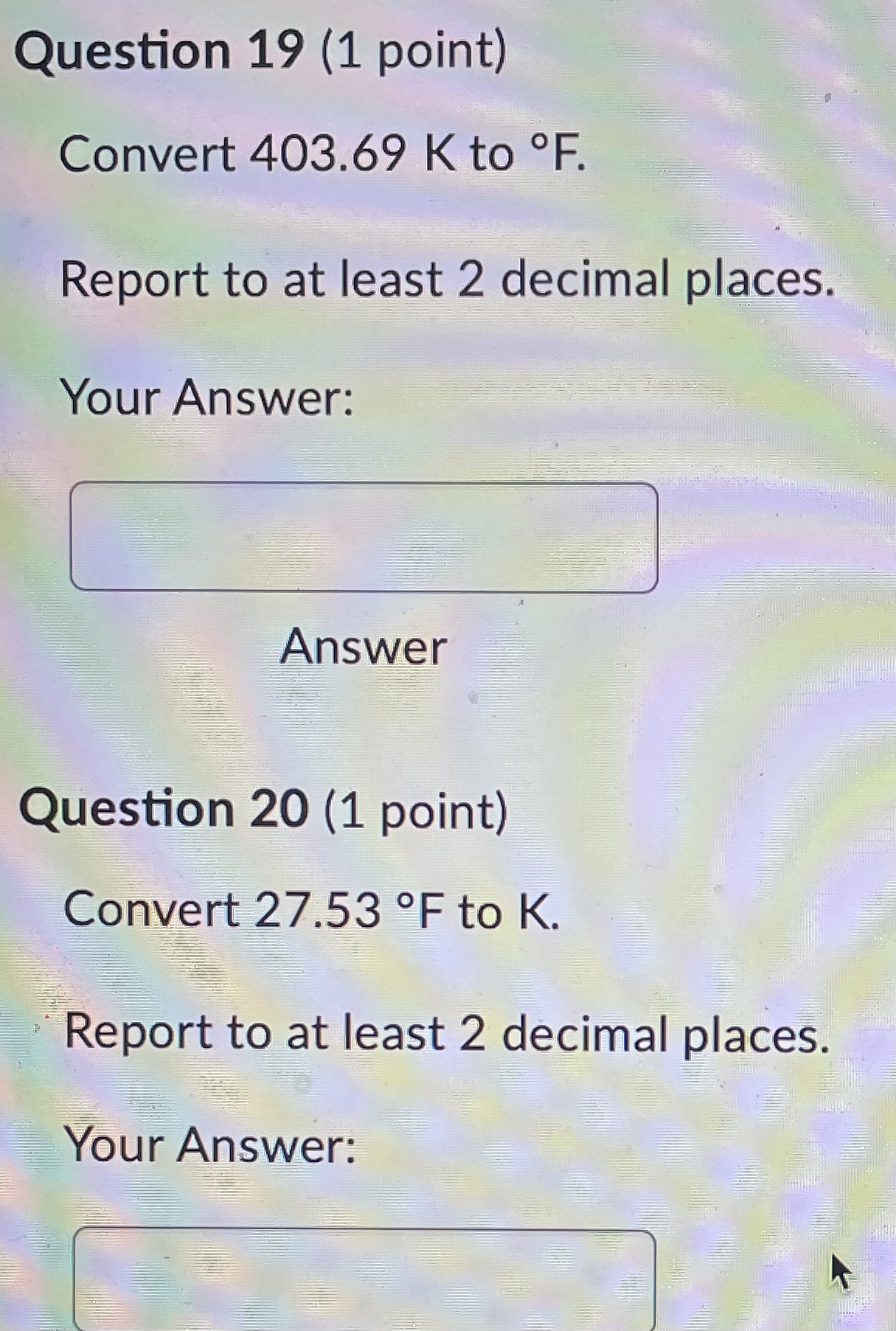
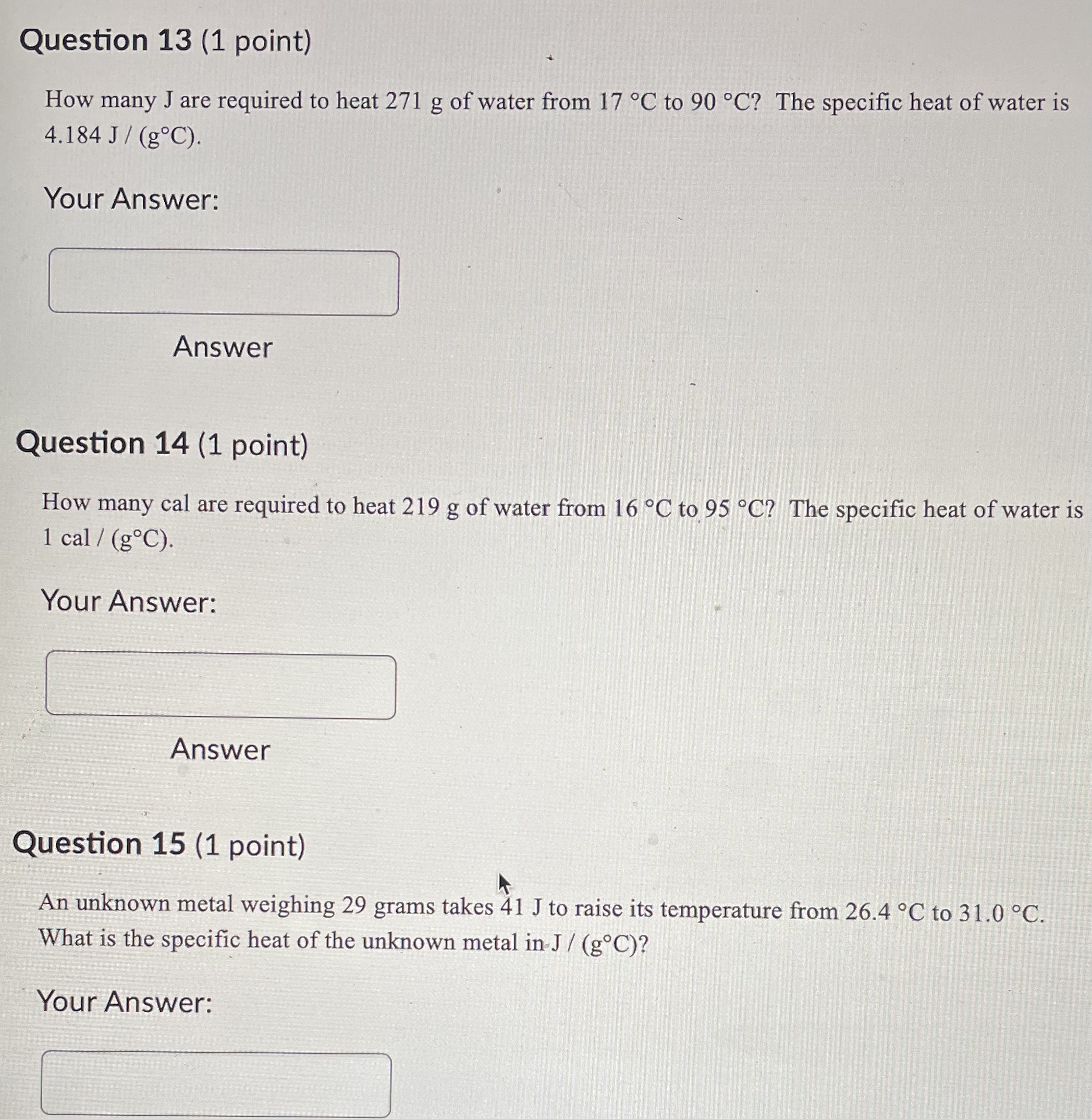
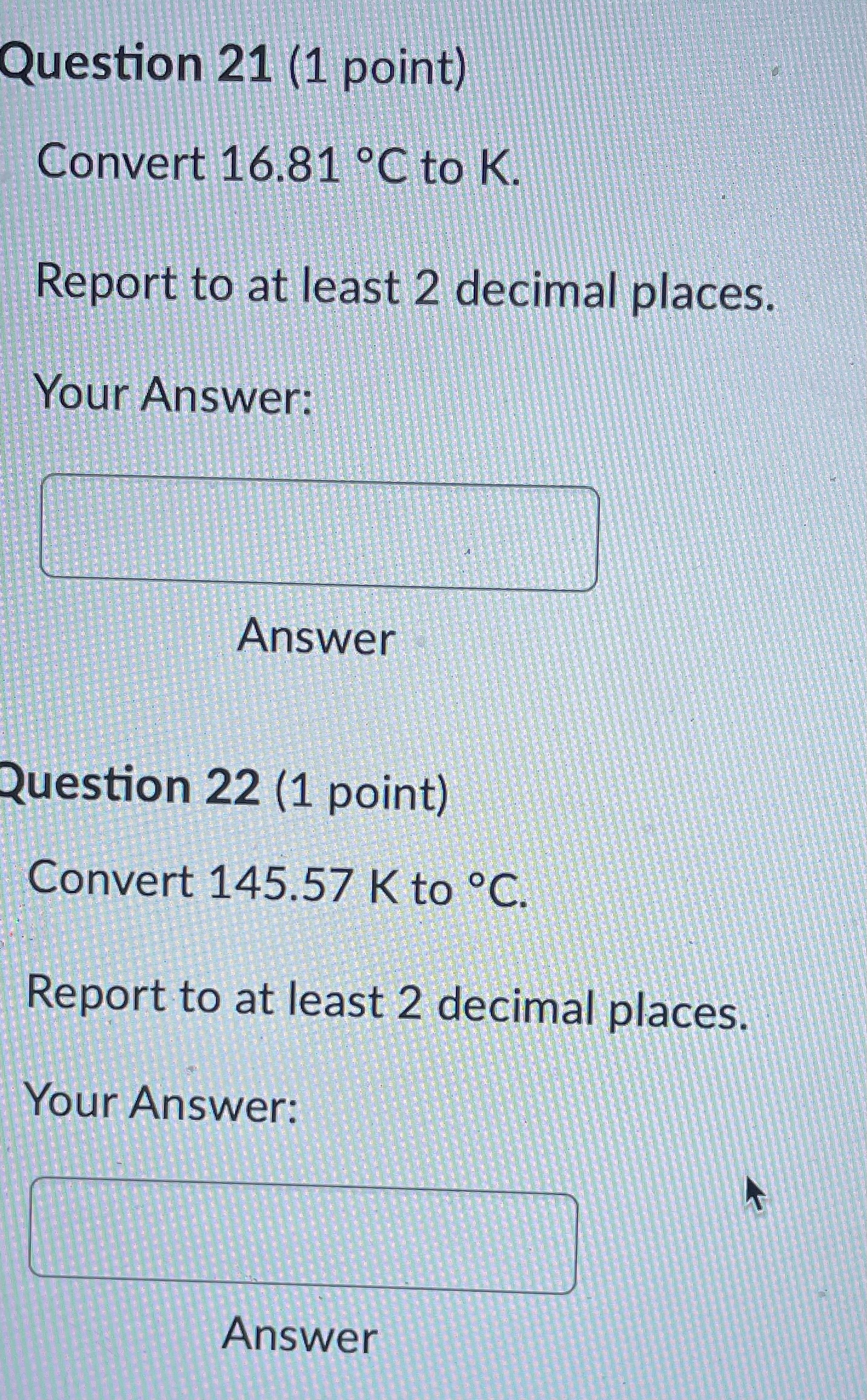
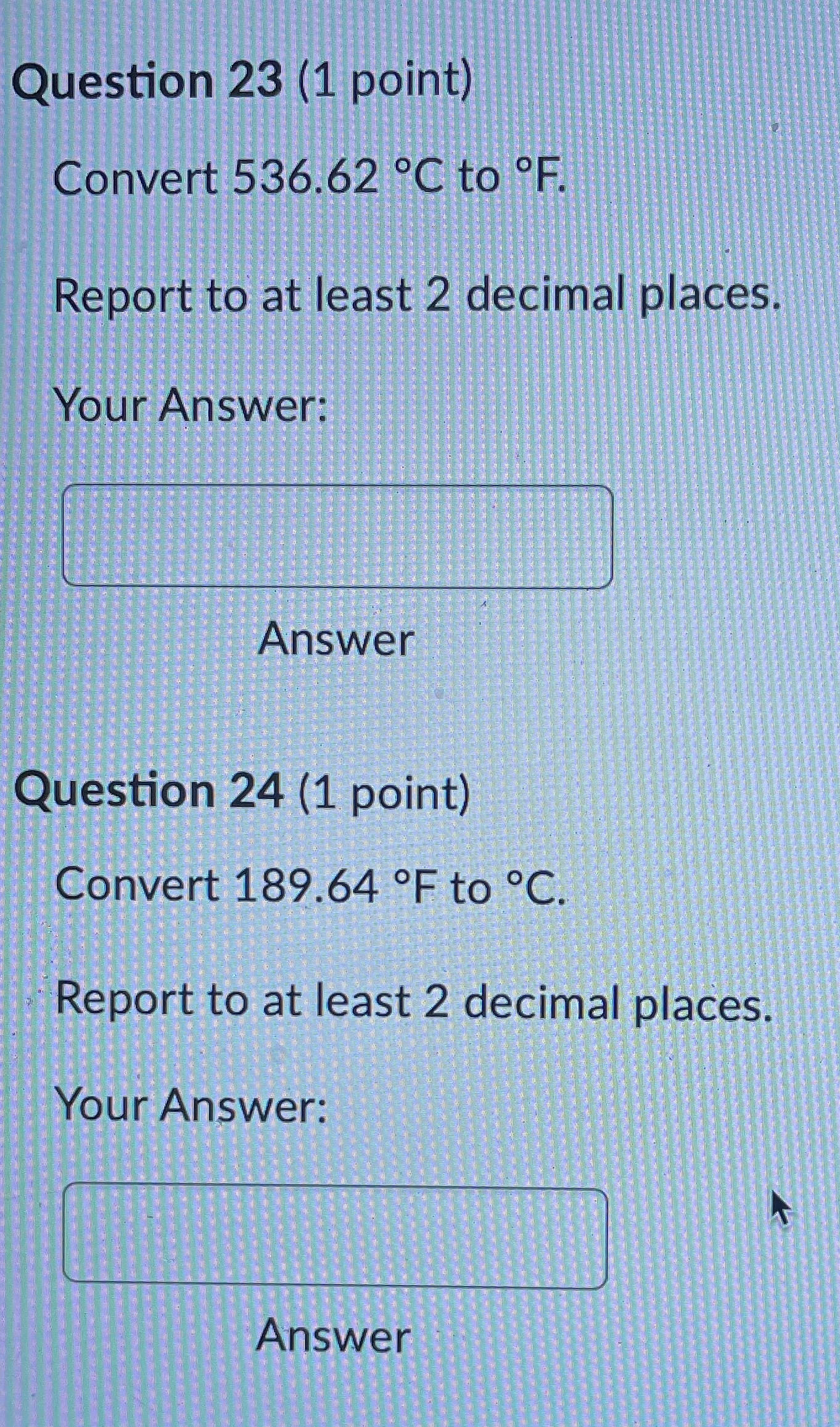

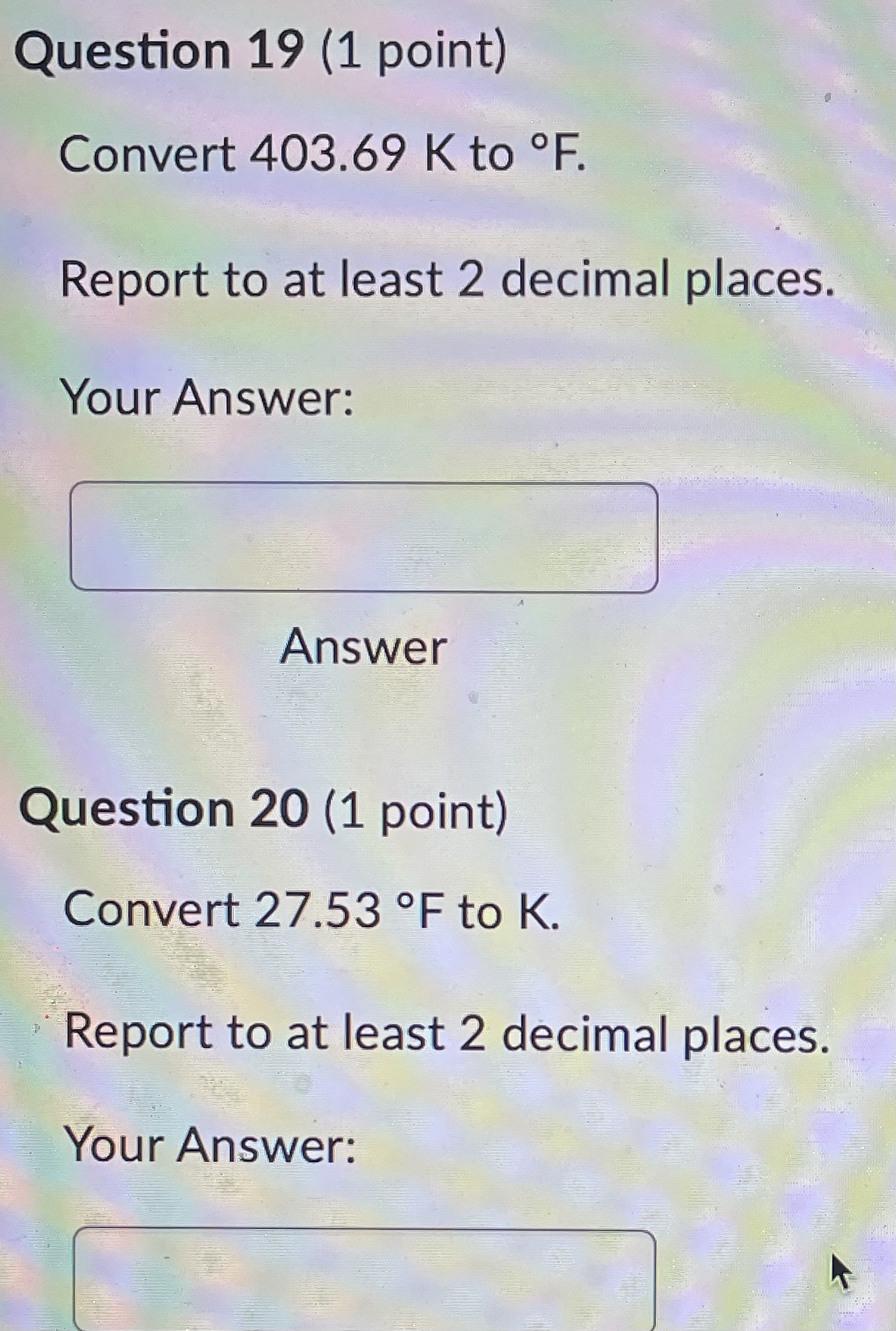
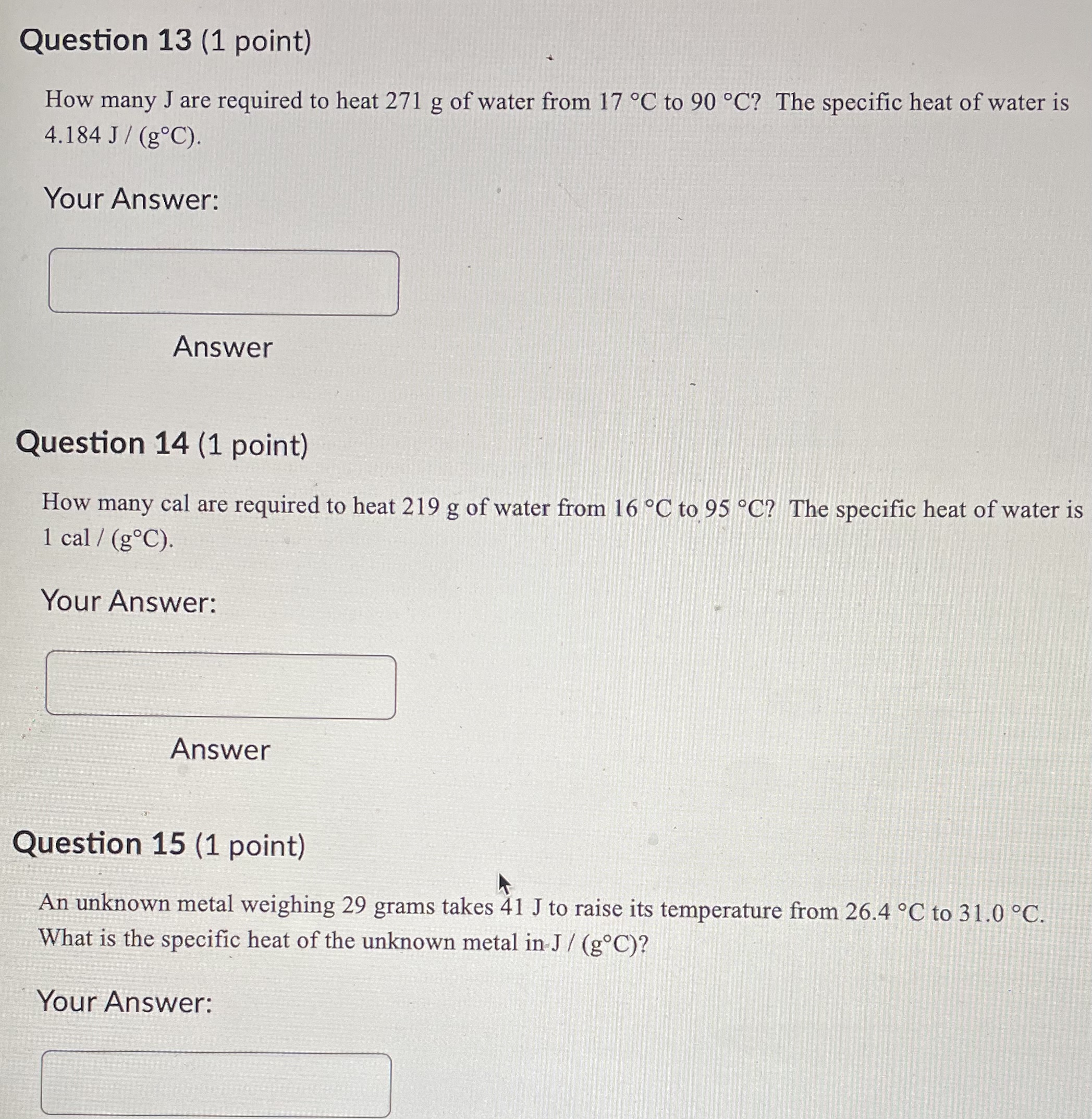
Question 21 (1 point) Convert 16.81 .C to K. Report to at least 2 decimal places. Your Answer: Answer Question 22 (1 point) Convert 145.57 K to C. Report to at least 2 decimal places. Your Answer: AnswerQuestion 23 (1 point) Convert 536.62 .C to OF. Report to at least 2 decimal places. Your Answer: Answer Question 24 (1 point) Convert 189.64 F to C. Report to at least 2 decimal places. Your Answer: AnswerQuestion 25 (1 point) Consider an iron rod that is 1-m long and expands 1/4 cm when heated. By how many cm will a 100-m rod of the same material expand when similarly heated? Your Answer: AnswerQuestion 19 (1 point) Convert 403.69 K to F. Report to at least 2 decimal places. Your Answer: Answer Question 20 (1 point) Convert 27.53 OF to K. Report to at least 2 decimal places. Your Answer:Question 13 (1 point) How many J are required to heat 271 g of water from 17 C to 90 .C? The specific heat of water is 4.184 J / (8.C). Your Answer: Answer Question 14 (1 point) How many cal are required to heat 219 g of water from 16 C to 95 .C? The specific heat of water is 1 cal / (goC). Your Answer: Answer Question 15 (1 point) An unknown metal weighing 29 grams takes 41 J to raise its temperature from 26.4 .C to 31.0 .C. What is the specific heat of the unknown metal in-J / (g.C)? Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


