Question: QUESTION 21 For the reaction A + B + C, the following plot gives a straight line, with the indicated slope and y intercept. slope
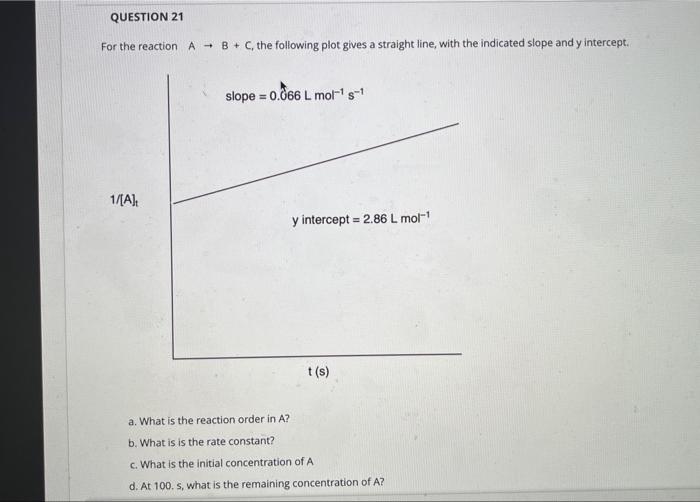
QUESTION 21 For the reaction A + B + C, the following plot gives a straight line, with the indicated slope and y intercept. slope =0.066 L mol's-1 1/[A] y intercept = 2.86 L mol-1 t(s) a. What is the reaction order in A? b. What is is the rate constant? c. What is the initial concentration of A d. At 100.5, what is the remaining concentration of A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


