Question: Question 2.2.6 Solid ruthenium (Ru) and osmium (Os) adopt the same crystal structure and have very similiar metallic atomic radii, 134pm and 135pm. respectively; however,
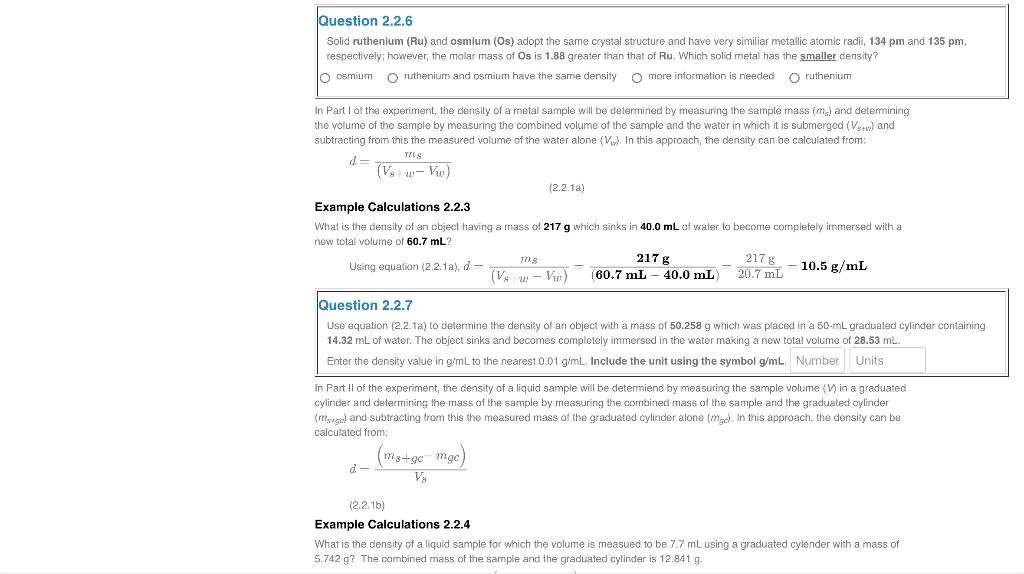
Question 2.2.6 Solid ruthenium (Ru) and osmium (Os) adopt the same crystal structure and have very similiar metallic atomic radii, 134pm and 135pm. respectively; however, the molar mass of Os is 1.88 greater than that of Ru. Which solid metal has the smaller density? osmium ruthenium and osmium have the same density more information is needed ruthenium In Part of the experiment, the density of a metal sample will be determined by measuring the sample mass ( ms ) and determining the volume of the sample by measuring the combined volume of the sample and the water in which it is submerged (Vs+w) and subtracting from this the measured volume of the water alone (Vv). In this approach, the density can be calculated from: d=(Vs+Vw)mus (2.2.1a) Example Calculations 2.2.3 new total volume of 60.7mL ? Usingequation(2.2.1a)+d(VgwVu)ms=(60.7mL40.0mL)217g20.7mL217g=10.5g/mL Question 2.2.7 Use equation (2.2.1a) to determine the density of an object with a mass of 50.258g which was placed in a 50mL graduated cylinder containing 14.32mL of water. The object sinks and becomes completely immersed in the water making a new total volume of 28.53mL. Enter the density value in g/mL to the nearest 0.01g/mL. Include the unit using the symbol g/mL. In Part Il of the experiment, the density of a liquid sample will be determiend by measuring the sample volume (V) in a graduated cylinder and determining the mass of the sample by measuring the combined mass of the sample and the graduated cylinder ( ms,g ) and subtracting from this the measured mass of the graduated cylinder alone ( mgod) in this approach, the density can be calculated from: dVy(m3+gcmgc)(2.2,1bj) Example Calculations 2.2.4 What is the density of a liquid sample for which the volume is measued to be 7.7mL using a graduated cylender with a mass of 5.742g ? The combined mass of the sample and the graduated cylinder is 12.841g. Question 2.2.6 Solid ruthenium (Ru) and osmium (Os) adopt the same crystal structure and have very similiar metallic atomic radii, 134pm and 135pm. respectively; however, the molar mass of Os is 1.88 greater than that of Ru. Which solid metal has the smaller density? osmium ruthenium and osmium have the same density more information is needed ruthenium In Part of the experiment, the density of a metal sample will be determined by measuring the sample mass ( ms ) and determining the volume of the sample by measuring the combined volume of the sample and the water in which it is submerged (Vs+w) and subtracting from this the measured volume of the water alone (Vv). In this approach, the density can be calculated from: d=(Vs+Vw)mus (2.2.1a) Example Calculations 2.2.3 new total volume of 60.7mL ? Usingequation(2.2.1a)+d(VgwVu)ms=(60.7mL40.0mL)217g20.7mL217g=10.5g/mL Question 2.2.7 Use equation (2.2.1a) to determine the density of an object with a mass of 50.258g which was placed in a 50mL graduated cylinder containing 14.32mL of water. The object sinks and becomes completely immersed in the water making a new total volume of 28.53mL. Enter the density value in g/mL to the nearest 0.01g/mL. Include the unit using the symbol g/mL. In Part Il of the experiment, the density of a liquid sample will be determiend by measuring the sample volume (V) in a graduated cylinder and determining the mass of the sample by measuring the combined mass of the sample and the graduated cylinder ( ms,g ) and subtracting from this the measured mass of the graduated cylinder alone ( mgod) in this approach, the density can be calculated from: dVy(m3+gcmgc)(2.2,1bj) Example Calculations 2.2.4 What is the density of a liquid sample for which the volume is measued to be 7.7mL using a graduated cylender with a mass of 5.742g ? The combined mass of the sample and the graduated cylinder is 12.841g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


