Question: Question 24 2 H2(g) + O2(g) + 2 H2O(1) AGran = -474 kJ/mol en Electrode Half-Reaction Standard Reduction Potential (V) ? Cathode 02(9) + 4H+
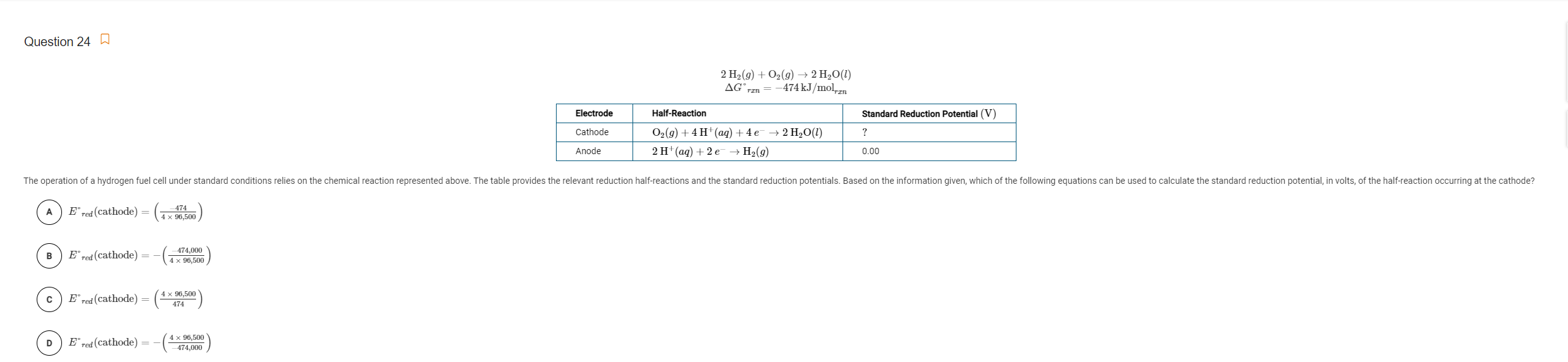
Question 24 2 H2(g) + O2(g) + 2 H2O(1) AGran = -474 kJ/mol en Electrode Half-Reaction Standard Reduction Potential (V) ? Cathode 02(9) + 4H+ (aq) + 4e + 2 H2O(1) 2 H(aq) + 2 H (9) Anode 0.00 The operation of a hydrogen fuel cell under standard conditions relies on the chemical reaction represented above. The table provides the relevant reduction half-reactions and the standard reduction potentials. Based on the information given, which of the following equations can be used to calculate the standard reduction potential, in volts, of the half-reaction occurring at the cathode? A Ered (cathode) = 474 4 x 96,500 B Ered (cathode) = 474,000 4 x 96,500 Ered (cathode) = (* X PCIE D Ered (cathode) = 4 x 96,500 474,000
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


