Question: Question 3 (1 point) Salcium carbide (CaC2) is produced when CaO, known as quicklime, reacts whth fon at high temperature. CaO(s)+3C(s)CaC2(s)+CO(g)1H=+480kJ/mol If 11.7g of carbon
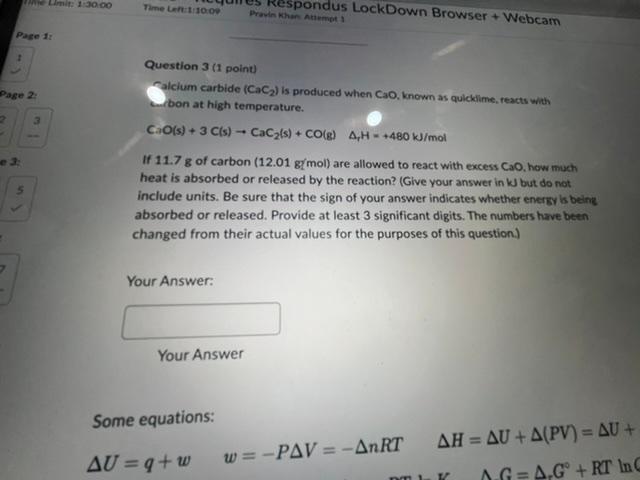
Question 3 (1 point) Salcium carbide (CaC2) is produced when CaO, known as quicklime, reacts whth fon at high temperature. CaO(s)+3C(s)CaC2(s)+CO(g)1H=+480kJ/mol If 11.7g of carbon (12.01g(mol) are allowed to react with excess CaO. how much heat is absorbed or released by the reaction? (Give your answer in k but do not include units. Be sure that the sign of your answer indicates whether energy ls being absorbed or released. Provide at least 3 significant digits. The numbers have been changed from their actual values for the purposes of this question.) Your Answer: Your Answer Some equations: U=q+ww=PV=nRTH=U+(PV)=U+
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


